Abstract
Aims: To test the efficacy and safety of a chitosan pad for femoral haemostasis as an adjunct to manual compression. Haemostasis of the femoral artery after coronary angiography by manual compression is time consuming and uncomfortable for the patient. Closure devices are costly and do not reduce vascular complication rate. The HemCon® pad is used by the US army to control traumatic bleeding. It consists of chitosan, a positively charged carbohydrate that attracts the negatively charged blood cells and platelets and promotes clotting.
Methods and results: Patients undergoing percutaneous coronary angiography were 1:1 randomised for manual compression with regular or HemCon® pad. All patients were catheterised with 6 Fr sheath and received 2500 u of heparin. Time to haemostasis, incidence of minor and major bleeding, haematoma size, post-procedural stay at the hospital and level of satisfaction were compared between the two groups. Seventy patients in the HemCon group and 66 patients in the regular pad groups were recruited. Activated clotting time (ACT) before manual compression was similar, 183.9±43.4 and 178.3±34.2 seconds in the HemCon® and regular pad groups respectively. Time to haemostasis was 5.6±2.1 and 8.4±3.5 minutes in the HemCon® and regular pad groups, respectively (p<0.001). Haematoma developed in 6% and 14.8% of patients in the HemCon® and regular pad group, respectively (p=0.14).
Conclusions: The HemCon® pad significantly decreased time-to-haemostasis compared to the regular pad. The total incidence of haematoma tended to be lower in the HemCon® pad compared to the regular pad group.
Introduction
Most coronary diagnostic and therapeutic procedures are performed via the femoral artery. After completion of the procedure, haemostasis of the artery is achieved by applying local pressure either manually or by mechanical devices. This is uncomfortable for the patient, time consuming for the physician and occasionally is complicated with vascular events that increase morbidity and hospital duration and reduce patient satisfaction1. Closure devices have shortened pressure time but they are costly and do not reduce the vascular complication rate2-4.
The HemCon® pad (HemCon Medical Technologies, Inc., Portland OR, USA) is used routinely in the US and other countries’ armies to control traumatic bleeding5. It consists of a carbohydrate called chitosan (Figure 1) produced by deacetylation of chitin, which is extracted from the shells of shrimps, lobsters and beetles. The positively charged chitosan molecules attract the negatively charged blood cells and platelets, thus promoting clotting.
The aim of the present trial was to study the efficacy of the HemCon® pad as an adjunct to manual compression to reduce time-to-haemostasis after percutaneous coronary angiography.
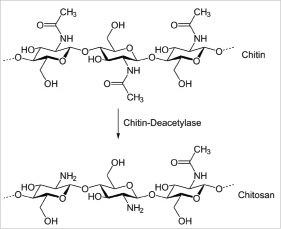
Figure 1. The chitosan molecule. The chitosan molecule is produced by deacetylation of chitin.
Methods
This was a single center, randomised, open label, placebo-controlled study.
Endpoints
The primary efficacy endpoint was comparison of time to haemostasis of HemCon® to regular pads. Secondary endpoints included vascular complication rate and patients’ satisfaction regarding time to sitting incline and time to ambulation.
Patients
Included in the study were 18-80 year-old patients undergoing diagnostic coronary angiography with a 6 Fr sheath through the right femoral artery. All patients received intravenous 2500 u of Heparin after insertion of the introducer into the femoral artery, and the contrast medium used was Iopromide.
Excluded were patients who had systolic blood pressure >150 mm Hg prior to sheath removal; who received >2500 U Heparin, LMWH or platelet glycoprotein IIb/IIIa antagonists eight hours before or during the angiography; with known bleeding tendency, disturbed clotting system or platelet function; and patients with evidence of bleeding or haematoma at the access site prior to sheath removal.
The study was approved by the hospital ethical committee and all patients gave written informed consent for participation in the trial.
Study protocol
HAEMOSTASIS
After completion of coronary angiography a 1:1 randomisation to HemCon® or regular (control) pad was performed.
Before the removal of the arterial sheath, a blood examination was taken for activated clotting time (ACT) and the result was recorded without affecting sheath removal timing or procedure.
The femoral sheath was removed by one of three senior operators at the patient’s bed immediately after the procedure. One to 2 ml of blood was allowed to exit from the access site and pressure with a HemCon® or regular pad (according to randomisation) was then applied manually for five minutes. After five minutes the operator released the pressure. If the bleeding continued, application of local pressure was resumed. After this stage, the operator was allowed to release the pressure after every five minutes or a longer (but not shorter) period of time depending on his impression and sense after first release. The total number of pressure releases and the total time to haemostasis were recorded.
After achieving haemostasis a pressure bandage was applied at the access site as practiced routinely in our hospital.
BED REST AND AMBULATION
Minimal bed rest duration was two hours. Total bed rest time was recorded.
IN HOSPITAL FOLLOW-UP
– A blood count was taken on the morning following the procedure.
– Haematomas were measured and recorded. Our definition for major haematoma was stricter than used in the literature6 and included a decrease in haemoglobin of ≥3.0 g/dl or need of any blood transfusion.
– Twenty five percent of all patients were randomly assigned to a duplex examination of the femoral artery at the access site performed both 3-4 hours after sheath removal and the day after the procedure.
DISCHARGE
Patients were routinely discharged one day after the procedure. Complications or adverse events were noted at the patient’s medical file and recorded in the clinical research form on discharge.
OUT OF HOSPITAL FOLLOW-UP
One day after discharge patients were contacted by phone and asked for new procedural complications such as groin pain or haematoma, and if needed were invited to the hospital for further examination. During the phone call patients were asked also of their satisfaction of the ambulation procedure at the scale of 1-10 (10 mostly satisfactory).
Statistics
With the assumption that the HemCon® pad will decrease time to haemostasis (the primary endpoint) by 30%, the calculated number of patients needed for the study (with alpha 0.05 and power 0.8) was 278.
The following data were compared between the two groups: Patients’ demographics (age and gender), BMI, coronary angiography duration in minutes, time to haemostasis, bed rest duration, incidence of any haematoma and of major haematoma, haematoma size, post procedural stay at the hospital, and level of satisfaction evidenced in post-procedure satisfaction surveys.
Patients’ data was stored on an Access database. Chi-square tests were used for categorical variables and independent sample 2-sided t-test for continuous variables. Analyses were performed using SPSS-17 software (SPSS Inc., Chicago, IL, USA). P<0.05 was used to determine statistical significance.
Results
The study was stopped after recruiting 136 patients (70 in the HemCon® and 66 in the control group) because an interim analysis showed the mean time to haemostasis was significantly lower with HemCon® compared to the regular pad group.
All patients received their assigned treatment with no crossovers.
There were no significant difference in demographic data, BMI, medications given before angiography and risk factors between the HemCon® pad and the regular pad groups (Table 1).
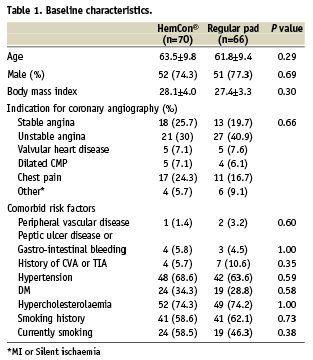
Procedural data after angiography are given in Table 2. There was no difference between the two groups in procedure duration, and in blood pressure and ACT upon arterial sheath removal.
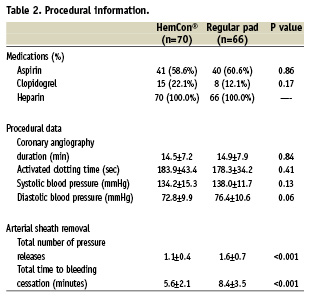
Haemostasis following sheath removal was achieved after five minutes in 90% and 43.9% in the HemCon® and control groups respectively (p<0.001) (Figure 2). The mean time to haemostasis following sheath removal was lower with the HemCon® pad by 33% compared to the conventional manual compression (5.6±2.1 vs. 8.4±3.5 minutes, p<0.001).
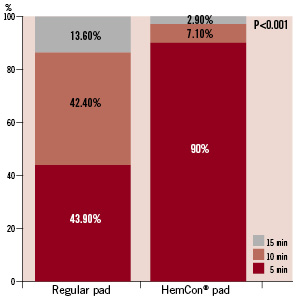
Figure 2. Distribution of time to haemostasis in the HemCon and the regular pad groups. Most patients in the control (regular pad) group needed 10-15 minutes of manual compression in order to achieve haemostasis of the femoral artery. After five minutes of manual compression 90% of patients in the HemCon group achieved femoral haemostasis compared to 43.9% in the control group (p<0.001).
Clinical outcomes are given in Table 3. There was no major haematoma or bleeding event in either group. Minor haematomas occurred in four patients (6%) in the HemCon group and in nine patients (14.8%) in the control group (p=0.14) without significant drop in haemoglobin and without need for blood transfusion. There was no bleeding after discharge in either group.
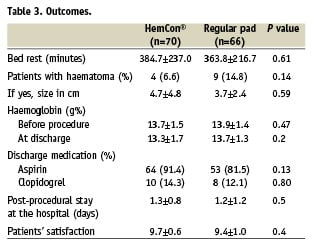
There was no difference in time to ambulation and in post-procedural stay at the hospital between the two groups, and there was no difference between the two groups regarding their satisfaction from the procedure of sheath removal and follow-up.
Twelve patients in the HemCon and eight patients in the control group were elected randomly for duplex examination of the femoral artery. No patient in the HemCon group and one patient in the regular pad group had pseudo-aneurysm.
Discussion
Local vascular complications at the site of sheath insertion are among the most common problems seen in cardiac catheterisation. These include acute vascular dissection, pseudo-aneurysm, arterio-venous fistula, thrombosis with or without distal embolisation and poorly controlled bleeding manifested as a free haemorrhage or contained haematoma in the thigh or retroperitoneal space. Haemorrhage and haematoma are usually evident within 12 hours after the procedure. The risk for adverse events in an individual patient is dependent on his demographics, cardiovascular anatomy, comorbidities, type of procedure performed and the experience of the operator1,7-16.
The traditional method for haemostasis at the access site is local hand-applied pressure17. Mechanical clamp pressure saves staff time and reduces vascular complications18 but might be painful and uncomfortable for the patient and does not decrease the need of staff attendance to ensure that the device remains in the correct position. Closure devices seal the arterial puncture with plug of collagen and polymer, sutures and the like19-23. They eliminate the need for prolonged arterial compression and reduce the interval of bed rest. Increased risk of local infection or endarteritis by closure devices, a rare but ominous complication has been reported (0.3% in one series)3,4. Two recent propensity analyses have shown trends that were either neutral24 or favoured vascular closure devices25. However these were single site studies at hospitals with extensive experience with vascular closure. To date, there have been no large scale randomised clinical trials comparing the outcomes of using vascular closure devices to manual compression.
The use of topical haemostasis patches as adjuncts to manual compression has grown rapidly over the last decade but the evidence-based data on their clinical benefit is limited. Some of these patches incorporate thrombin or calcium while others include a polysaccharide-based pro-coagulant. In the study by Narins et al, time to haemostasis was improved but this did not translate into a clinically meaningful difference26. Recently, chitosan – a polymer of poly-d-glucosamine and poly-n-acetyl-d-glucosamine found in the shells of shrimp, lobster and beetles have been shown effective in promoting haemostasis27,28. The chitosan positively charged molecules attract the negatively charged blood cells and platelets thus promoting clotting. The HemCon® pad is composed of chitosan. Preclinical surgical and trauma models29-31 have been successfully treated with the HemCon® bandage. Chitosan-based haemostatic dressing has been found effective in controlling bleeding related to traumatic battle-field injuries5,32-33. US military personnel in the Iraqi and Afghanistan conflicts have used the HemCon® bandage to successfully stop arterial haemorrhage not controlled by conventional bandages in 64 patients without adverse effects34,5. The HemCon® bandage is also an FDA-cleared haemostatic dressing suitable for use in haemodialysis access puncture35.
Retrospective analysis of patients with patches containing poly-n-acetyl-d- glucosamine36 and thrombin37 undergoing cardiac catheterisation or EP study demonstrated low incidence of major or minor vascular complications, but were not compared to conventional manual compression. A randomised study in patients undergoing PCI demonstrated a significant reduction in time to haemostasis with the use of either of two chitosan-based patches compared to manual compression alone38. Patients undergoing diagnostic procedures were not included in the study. Another randomised study in PCI patients used a haemostasis patch with a polysaccharide based pro-coagulant (SafeSeal® patch; Medrad, Inc., Warrendale, PA, USA). Time to haemostasis was somewhat lower in the haemostasis patch arm (11.8±3.6 versus 13.8±5.8 minutes)26.
In the present study we found that the HemCon® pad significantly decreased time to haemostasis by 33% from 8.4 to 5.6 minutes. Although the absolute decrease in time is not dramatic, it is meaningful in terms of shortening the painful manual pressure application which is frequently the most unpleasant part of the whole experience. Saving 2.8 minutes per patient is also significant for a busy operator who performs 10 procedures a day. The number of patients with mild haematoma at discharge tended to be lower in the HemCon group compared to regular pad. Although mild haematoma has negligible clinical significance, it is sometimes the only thing the patient remembers. Duplex examination of the femoral artery performed in a small group of patients excluded vascular complications such as subcutaneous haematoma, pseudo-aneurysm and arterial-venous fistula.
Although this clinical trial was performed only in patients who underwent diagnostic angiography, the intravenous infusion of 2,500 units of heparin included in the protocol increased the ACT to 180 seconds. This implies that our results may be extended to patients undergoing interventional procedure with higher doses of heparin provided the ACT is checked and allowed to decrease below 200 seconds. This assumption needs to be substantiated in future clinical trials.
Patient’s satisfaction was very high in both groups and therefore did not disclose any difference between the groups.
Patients’ demographics, risk factors and medications taken prior to angiography in this study represent the patients population who undergo diagnostic coronary angiography with no angioplasty at our laboratory (most of them with non-significant coronary disease), and there was no difference between the HemCon® and regular pad groups.
The trial’s planned sample size was 139 subjects per treatment arm. During the trial, HemCon’s superiority to regular pads became apparent and it was decided to compare the arms earlier than planned. The statistical test yielded a very significant result (p<0.001) for both mean time to haemostasis and percentage of patients achieving haemostasis after five minutes. Based on the statistical guidelines of the International Conference on Harmonisation (ICH), we recommended that the trial under the current exploratory protocol be discontinued and its conclusion would be that HemCon® is superior to regular pads in this indication.
Limitations
The open label design could bias the results because the operators were aware of the pad used for haemostasis. Since the HemCon® pad is composed totally of chitosan, it looks different from regular pads and it would be very difficult to produce a placebo pad for pursuing a double blinded study. The HemCon® pad was used as an adjunct to manual compression and the intensity of manual compression was not controlled. However, complications were rare and this factor did not affect our results. The similarity between groups regarding time to discharge is a reflection of our practice to observe patients until the day after the diagnostic catheterisation. Early time to discharge is relevant for practices that discharge patients within a few hours, and should be addressed in a different study. We did not perform cost-benefit analysis because the HemCon® pad was not yet marketed in our country and its price has not been determined.
In summary, the HemCon® pad significantly decreased time-to-haemostasis compared to regular pads in patients undergoing percutaneous femoral angiography. The total incidence of haematoma tended to decrease in the HemCon® pad compared to the control group.
Excerpt from a reviewer
The authors present a randomised open label trial using chitosan vs. manual compression for diagnostic cardiac catheterisation. The sample size appears to be adequate to prove the primary endpoint which was time to haemostasis. The difference (roughly 2.8 minutes) is statistically significant; however, the clinical relevance of this study is limited since time to ambulation or time to discharge did not vary between groups. The similarity between groups regarding time to discharge may only be a reflection of the local practice where patients appear to be observed for 24 hours after diagnostic catheterisation, but early time to ambulation or discharge may be a more clinically relevant endpoint especially for practices that require discharging patients a few hours after a diagnostic catheterisation.

