
Abstract
Aims: Haemostasis is a limiting factor for discharge after uncomplicated transradial procedures. The purpose of this study was to determine whether a potassium ferrate haemostatic patch (PFHP) could serve as an adjunct to the air-bladder TR Band (TRB) to facilitate implementation of a rapid deflation protocol.
Methods and results: This was a prospective multicentre randomised controlled trial comparing radial haemostatic protocols. Deflation of the TRB was attempted at 40 minutes with PFHP and at 120 minutes without the PFHP. The primary outcome was time to full deflation of the TRB with haemostasis. At four US sites, 180 patients were enrolled after receiving a minimum of 5,000 units of unfractionated heparin or bivalirudin. Interventions comprised 30% of procedures. Successful TRB deflation occurred at 43±14 minutes with PFHP and 160±43 minutes without PFHP (p<0.001). Minor haematomas occurred in nine (10.3%) of the TRB patients and 16 (17.2%) of the PFHP patients (p=0.20). Radial artery occlusion occurred in 2% of patients in the PFHP group (p=NS). Outpatients randomised to PFHP were discharged 51±83.5 minutes earlier than control.
Conclusions: The PFHP haemostatic patch facilitated early deflation of the TRB with a non-significant increase in forearm haematomas. Use of the PFHP may improve patient throughput and allow earlier discharge following transradial procedures. ClinicalTrials.gov: NCT03028025
Abbreviations
PFHP: potassium ferrate haemostatic patch
RAO: radial artery occlusion
TRB: TR Band
Introduction
Transradial catheterisation is associated with reduced vascular complications, improved patient satisfaction, and cost efficiencies compared with femoral access1,2. Haemostasis following transradial procedures is achieved through either prolonged manual compression or use of compression devices such as air-bladder bracelets (TR Band® [TRB]; Terumo Corp., Tokyo, Japan)3.
Despite their frequent use, there is no consensus on the appropriate duration of compression with radial haemostatic devices. For the TRB, there are no manufacturer recommendations and current practice varies widely, with two hours of compression frequently used for diagnostic procedures and up to four hours of compression for interventions. Reduction in compression times may reduce the need for observation in the recovery area, reduce the risk of radial artery occlusion (RAO), and facilitate patient throughput and earlier discharge following transradial diagnostic or interventional procedures.
The purpose of this study was to determine whether a potassium ferrate haemostatic patch (PFHP) (StatSeal® Advanced; Biolife, LLC, Sarasota, FL, USA) could serve as an adjunct to the TRB to facilitate implementation of a rapid deflation protocol. The PFHP disc contains potassium ferrate on a bed of hydrophilic polymer. The polymer rapidly dehydrates and concentrates the blood solids, while the potassium ferrate agglomerates the solids and proteins together, forming a seal to the wound to stop bleeding and oozing, and preventing any transmission of the potassium ferrate material below the skin. Beneath the seal, the blood solids and proteins continue to stack naturally down, potentially facilitating haemostasis at the arteriotomy site without components of the PFHP reaching the vessel.
Methods
STUDY DESIGN
The Statseal with TR Band assessment trial (STAT) was a prospective, multicentre, randomised, open-label controlled trial comparing the PFHP patch in conjunction with the TRB versus the TRB alone. The study was investigator initiated and designed. A sample size of 180 patients was calculated in order to provide >95% power to detect an estimated 60-minute difference in time to TRB deflation. The study sample size of 180 patients was designed to enable four different sites to enrol patients, with a minimum contribution of 30 patients and a maximum of 60 from each site. All patients provided written informed consent for the research study, and the study was approved by the institutional review board of each institution. Patients were randomised in a 1:1 fashion using sealed envelopes balanced in blocks of 30 generated for each centre by a central coordinating centre. Patients were not randomised until their catheterisation procedure was completed and the sheath ready to be removed.
ELIGIBILITY CRITERIA
Adult patients presenting for transradial cardiac catheterisation procedures (diagnostic or interventional) were included in the trial. Patients with sheaths larger than 6 Fr diameter, chronic oral anticoagulation continued during the procedure, ipsilateral arteriovenous fistula, clinical instability, Barbeau’s class D radial plethysmography, known vascular disease or deformity of the forearm, or history of RAO at baseline were excluded from the study.
STUDY PROTOCOL
All patients received baseline assessment of the hand circulation using Barbeau’s test4. All procedures were performed according to local standards and operator preference, with the exception of the haemostasis protocol. All procedures were performed with short (10 cm) 4-6 Fr hydrophilic sheaths (Glidesheath and Glidesheath Slender®; Terumo Corp.), and subcutaneous lidocaine as per local practice. A single- or double-wall puncture technique was used per operator preference. A minimum of 5,000 units of intravenous unfractionated heparin (UFH) or weight-based bivalirudin was required for anticoagulation. Vasodilators for radial artery spasm prophylaxis were administered as per local practice.
For patients randomised to the control group of TRB alone, the band was applied over the arteriotomy site and inflated with 15-18 ml of air, and the radial sheath removed. The TRB was then deflated until bleeding occurred, and 2 ml of air reintroduced to provide “patent” haemostasis. The band was left inflated for two hours following the procedure for all patients, after which attempts at complete deflation began.
For patients randomised to PFHP with TRB, following catheterisation the PFHP was applied to the arteriotomy site, and a waterproof dressing (Tegaderm™; 3M, St. Paul, MN, USA) applied over the PFHP and sheath. The TRB was applied over both and inflated to a fixed 8 ml of air inflation, and the sheath removed (Figure 1, Moving image 1). In general (>95% of the time), this was sufficient inflation to create occlusive or haemostatic pressure, but 1-2 ml additional air was added if initial haemostasis was inadequate. After 20 minutes, 3 ml of air was removed with the goal of providing patent haemostasis. At 40 minutes, the TRB was fully deflated but left in place around the wrist to discourage movement. At 60 minutes, the TRB was removed, leaving the PFHP device remaining over the arteriotomy. Patients were instructed to remove the dressing and PFHP after 24 hours.
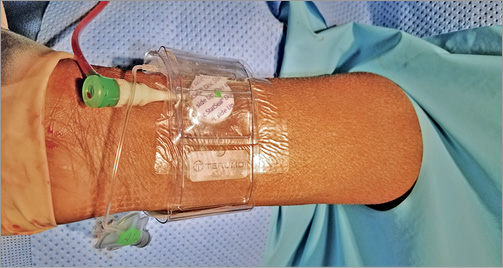
Figure 1. Rapid deflation protocol with the PFHP. The PFHP is placed over the radial arteriotomy site, covered with a sterile dressing, and the TR Band applied. The TR Band is inflated to a fixed 8 cc of air and the sheath removed. The band is deflated by 3 cc after 20 minutes and fully deflated at 40 minutes.
DATA COLLECTION
Baseline patient demographics, comorbidities, and laboratory values were recorded. Procedural details including number of puncture attempts, medications, sheath size, type of procedure, access technique (single- or double-wall), and blood pressure at the conclusion of the procedure were also recorded. The kaolin activated clotting time (ACT) was measured at least five minutes following dosing with unfractionated heparin.
Radial artery patency was assessed with plethysmography and oximetry using the reverse Barbeau’s test. The radial artery plethysmography waveform was categorised as either present, diminished, or absent while the ulnar artery was compressed. In the TRB group, radial patency by plethysmography-oximetry was documented within five minutes after TRB application, at the time of TRB removal, and at discharge or 24 hours. In the PFHP group, radial patency was documented at five minutes, 20 minutes, 40 minutes, and 60 minutes or TRB removal. Discharge criteria were according to the usual care at each institution.
OUTCOME MEASURES
The primary outcome measure was time to full initial TRB deflation with successful initial haemostasis. The secondary outcomes included time to discharge for outpatients, radial artery patency, and haematoma formation. Haematomas were categorised according to the five grades of the EArly Discharge after Transradial Stenting of CoronarY Arteries (EASY) trial (<5 cm, 5-10 cm, forearm swelling, haematoma above the elbow, and compartment syndrome)5.
STATISTICAL ANALYSIS
Continuous data are presented as mean±standard deviation and as median (interquartile range), and compared using the unpaired t-test and Mann-Whitney U test, respectively. Discrete data are presented as frequencies with their respective percentages and compared using Fisher’s exact test. Outcome data were analysed in a per-protocol analysis. Two-tailed tests of significance are reported and p-values <0.05 were considered statistically significant. Univariate logistic regression was performed using all baseline patient and procedural variables available to predict the secondary outcome of haematoma formation. Variables that were significant in univariate analysis at p<0.25 were retained and entered into a multivariate model, with a threshold of p<0.05 being considered as significant. Statistical analyses were performed using the SPSS statistical software programme, Version 16.0.2 (SPSS Inc., Chicago, IL, USA).
Results
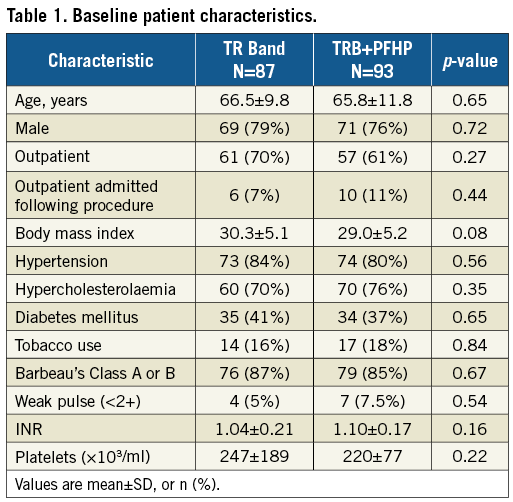
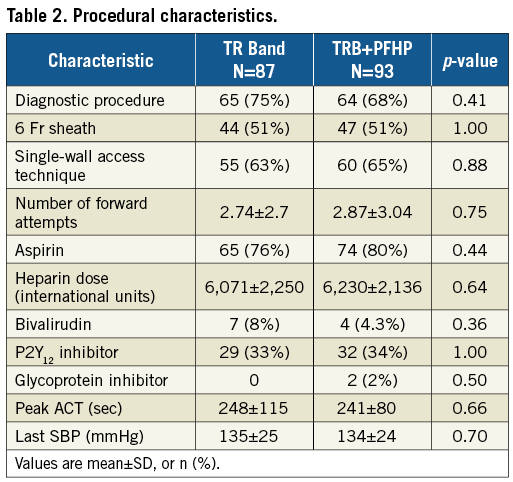
From January to August 2017, 184 patients were enrolled at four centres in the USA. Four patients (three TRB, one PFHP) were excluded from the analysis for not receiving the protocol-mandated minimum dose of heparin or bivalirudin during the procedure. Among the 180 included patients, 93 were in the PFHP group and 87 in the TRB alone group. The majority (78%) were male. There were no significant differences in baseline patient characteristics between groups (Table 1). Outpatients discharged the same day comprised 66% of the sample. There were also no differences in procedural characteristics (Table 2), including number of puncture attempts, adjunctive pharmacology, peak ACT, or blood pressure. Percutaneous coronary interventions (PCI) comprised 30% of the procedures. The single-wall puncture technique was used in 64% of cases.
OUTCOMES
The primary endpoint of mean time to full TRB deflation was significantly lower in the PFHP group as compared to the TRB control group (43±14 vs. 160±43 minutes, p<0.001). The mean difference between groups was 117±5.2 minutes (Table 3). TRB deflation was consistently achieved in the PFHP group regardless of single- or double-wall puncture technique (40.3±2.3 minutes and 44.7±19.4 minutes, p=0.38). In 55% of patients in the control group, the time required for deflation exceeded the minimum time of 120 minutes by over 30 minutes, most commonly due to the need for reinflation to control bleeding (Figure 2A).
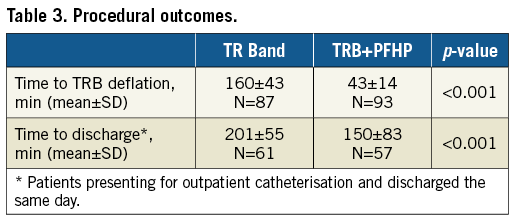
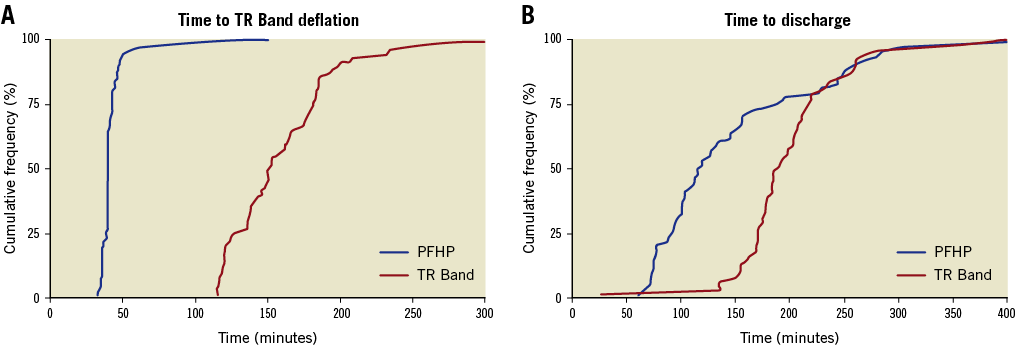
Figure 2. Time to TR Band deflation and discharge. A) Cumulative frequency plot of the time to successful TR Band deflation in patients receiving PFHP+TRB (blue, n=93) and TRB alone (red, n=87). B) Cumulative frequency plot of time to discharge amongst outpatients discharged the same day receiving PFHP+TRB (blue, n=57) and TRB alone (red, n=61).
Among those patients presenting for outpatient catheterisation and not admitted, the secondary outcome of time to discharge following the procedure was 51±83.5 minutes faster with the use of the PFHP. Patients randomised to the PFHP with TRB were discharged sooner after the procedure than those with TRB alone (150±83 minutes vs. 201±55 minutes, p<0.001) (Figure 2B).
Plethysmography-oximetry demonstrated occlusive pressures in 43% of patients with the PFHP at five minutes, but patency was largely restored on the initial deflation at 20 minutes (6% occlusive), and the remaining occlusions relieved by 40 minutes and one hour (with 98% of radial arteries patent). This was not significantly different from the final radial patency with the TRB alone. Radial artery occlusion occurred in 2% of patients in the PFHP group, compared to none in the TRB group (p=0.50) (Figure 3).
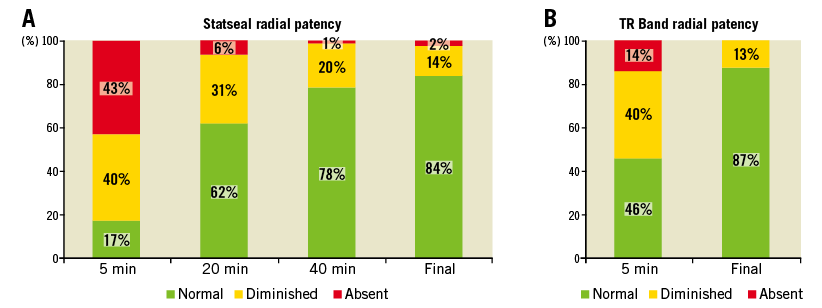
Figure 3. Radial artery patency during TR Band compression. Radial artery plethysmography was monitored at 5, 20, 40 minutes, and removal in patients randomised to PFHP+TR Band (A), and at five minutes and deflation/removal in TR Band alone patients (B). For each time point, following ulnar artery compression, the first digit arterial waveform was classified as normal (patent), diminished, or absent (occluded).
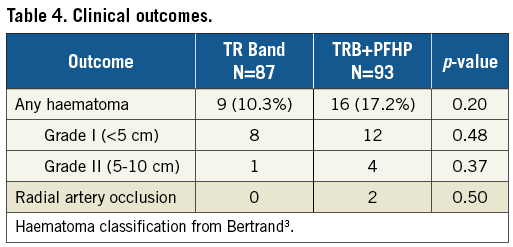
Minor haematomas occurred in nine (10.3%) TRB patients and 16 (17.2%) PFHP patients (p=0.20) (Table 4). The majority of these were <5 cm in size (80%). No haematomas >10 cm were noted. Haematomas were managed with prolonged compression with either manual pressure, additional TR Bands, or blood pressure cuffs applied around the forearm. Haematomas in two TRB and three PFHP patients occurred proximal to the TRB early during compression, indicating potentially inaccurate placement of the TRB over the arteriotomy or wire perforation of a small branch during initial access. Glycoprotein IIb/IIIa inhibitors were used in only two patients but were associated with haematomas in both cases. Univariate analysis did not demonstrate any significant association between haematoma formation and procedure type, puncture technique, sheath size, or number of puncture attempts. By univariate analysis, haematoma formation was associated with UFH total dose and inversely related to weight, international normalised ratio (INR) value, and platelet value. However, in multivariable analysis only weight (p=0.01) was inversely related to haematoma formation (Supplementary Table 1-Supplementary Table 3).
Discussion
In this multicentre randomised controlled trial, a rapid TRB deflation protocol for radial haemostasis was facilitated with the use of a PFHP. Compared with a standard deflation time of two hours with the TRB alone, the PFHP protocol enabled TRB deflation at 43 minutes and a 51-minute reduction in time to discharge. Minor forearm haematomas were numerically higher with the PFHP protocol, but there were no statistically significant differences in haematomas overall or in radial artery patency.
Compared with transfemoral access, transradial access reduces costs, vascular complications, and the need for overnight observation following PCI. However, there remain opportunities for further improvement in care pathways. Following transradial PCI, typical protocols utilise compression with the TRB for up to four hours or require multiple deflation steps, which is labour intensive, utilises high-value nursing resources, and results in increased costs. Reducing the compression and observation time following transradial catheterisation procedures can improve patient throughput and reduce costs, provided it can be performed without increasing complications of bleeding or thrombosis. Haemostatic agents such as the PFHP patch are potentially cost-saving as the price of such devices is probably less than the cost of an hour of nursing observation.
There is no consensus regarding the optimal duration of radial artery compression or the best device to use to achieve haemostasis and maintain radial artery patency. The air-filled TRB and similar devices are the most widely used due to ease of use, adjustability, and a characteristic gradual reduction in applied pressures over time. Compared with a six-hour compression time, a two-hour compression time with the TRB has been shown to reduce RAO6. Recently, a series of single-centre randomised trials (CRASOC I, II, III) demonstrated that a 90-minute compression was superior to a 2-, 3-, and 4-hour compression time to reduce the incidence of RAO; however, haematomas were more frequent with shorter compression7. Ultrashort compression times may have limits, with 20 minutes of compression showing higher rates of haematoma formation, increased need for recompression, and numerically increased rates of RAO compared with 60 minutes8.
The dose of UFH used in this study (5,000 IU) was based on prior studies indicating a reduction in RAO compared with 2,000-3,000 units, albeit with a trend towards increased minor bleeding9. However, many of these prior studies utilised prolonged compression times, occlusive haemostatic devices or both. The incremental benefit of higher doses of heparin in reducing RAO in the setting of patent haemostasis or rapid-deflation protocols is unclear and requires further study in randomised trials. A recent international survey of transradial operators indicated that 48% of those who use a fixed dose of UFH for diagnostic catheterisation use 5,000 units, with most others using between 2,000 and 5,000 units10. In one trial, outcomes with provisional heparin were equivalent to routine UFH (50 IU/kg) administration provided that plethysmography-guided patent haemostasis was achieved11. The current study indicating a low 1% rate of RAO is similar to other studies of short-duration compression7,8,12 and may indicate that, in the setting of early deflation and patent haemostasis, heparin doses can potentially be reduced or eliminated to minimise the risk of bleeding after diagnostic catheterisation. Any difference in RAO rates between the PFHP and the TRB within the baseline range of 0-2% can only be detected with sample sizes of several thousand patients and may not be clinically relevant.
The optimal duration of radial compression is probably a function of anticoagulation intensity and sheath size, with other minor factors potentially including blood pressure, radial artery size, compressive force, depth of artery below the skin, artery elasticity, and procedure duration. The primary reason for inability to deflate a TRB promptly following catheterisation is superficial re-bleeding7 and need for reinflation, which occurred in 55% of patients in this study despite the two-hour compression time. In contrast, patients receiving PFHP very rarely had superficial re-bleeding, indicating effective haemostasis at the skin-PFHP interface, enabling deflation and removal of the TRB. However, the numerical increase in haematoma formation in the PFHP group suggests that in some cases superficial haemostasis did not guarantee haemostasis at a deeper level at or below the arteriotomy site.
Adjustments to the PFHP protocol to reduce haematoma rates could include reducing the heparin dose for diagnostic procedures, increasing the duration of compression, modifying the size or shape of the patch to distribute compressive pressures proximally and more equally, and immobilising the wrist to minimise disruption of the haemostatic plug in the early phase following TRB removal. In conjunction with recent studies described previously, the optimal duration of compression with the TRB alone appears to be between 90 and 120 minutes6,7, while the PFHP potentially reduces this time to 40-60 minutes. Future studies of the PFHP would require approximately 800 patients to demonstrate non-inferiority in haematoma rates with an absolute non-inferiority margin of 2%.
Adjunctive topical haemostatic agents including thrombin (D-Stat; Vascular Solutions [now Teleflex], Minneapolis, MN, USA), kaolin-impregnated gauze (Quik-Clot®; Z-Medica, Wallingford, CT, USA), chitosan (Chito-Seal; Abbott Vascular, Santa Clara, CA, USA), polyprolate (Clo-Sur P.A.D.®; Merit Medical, South Jordan, UT, USA), and potassium ferrate (StatSeal) accelerate physiologic coagulation and clot formation by various pharmacologic, dessicant, and chemical actions. All have been tested in various models of bleeding, with the PFHP demonstrating some superiority over the thrombin and kaolin products13. There have been no reports of allergy, infection, or exothermic burns in response to the potassium ferrate material. The devices have had a limited role in femoral access procedures, possibly due to the typical 3-4 cm depth of the arteriotomy from the skin puncture. However, as the radial artery is only 0.5 cm below the skin, topical haemostatic agents may be more effective in accelerating haemostasis at the arteriotomy of transradial procedures. A recent pilot study suggested that the Quik-Clot product may accelerate transradial haemostasis14. Further study is warranted.
Limitations
The present study was not adequately powered for RAO or haematomas. The study was open-label and could not reasonably be blinded. Our protocol-defined primary endpoint of time to TRB deflation was subject to differential treatment, as patients with the TRB alone had a minimum compression time of 120 minutes rather than 40 minutes. This time was the minimal TRB compression time that was widely accepted at the time of study design based on clinical experience and prior studies6, and shorter compression times have subsequently been associated with bleeding7,8. As the majority of TRB patients exceeded the minimum time by over 30 minutes, the results were not likely to have differed had the initial deflation attempt been earlier following catheterisation.
Conclusions
The potassium ferrate haemostatic patch (PFHP) facilitated early TR Band (TRB) deflation and early discharge following transradial catheterisation, with a numerical but not statically significant increase in minor haematoma formation. Further studies will determine the optimal compression protocol to balance compression time and haematoma formation.
| Impact on daily practice The potassium ferrate haemostatic patch facilitated early deflation of the TR Band and early discharge and may improve the efficiency of diagnostic and interventional transradial procedures. |
Funding
The sponsor (Biolife) provided funds for IRB review at one out of our four sites; otherwise, there was no funding of this study, and the sponsor had no role in the design or conduct of the study, in the collection, analysis, and interpretation of the data, and in the preparation, review, or approval of the manuscript.
Conflict of interest statement
M. Cohen is a consultant for Biolife, the maker of the PFHP device, and a speaker for Terumo. The other authors have no conflicts of interest to declare.
Supplementary data
Supplementary Table 1. Prediction of haematoma formation: univariate logistic regression analysis.
Supplementary Table 2. Prediction of haematoma formation: multivariate logistic regression analysis.
Supplementary Table 3. Odds ratio of haematoma formation: multivariate logistic regression analysis.
To read the full content of this article, please download the PDF.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Application of the PFHP in conjunction with the TR Band following transradial catheterisation.

