
bstract
Aims: The 6 Fr Glidesheath Slender (GSS6Fr) is a recently developed thin-walled radial sheath with an outer diameter (OD) that is smaller than the OD of standard 6 Fr sheaths. The purpose of this trial was to clarify whether the use of this new slender sheath would result in similar rates of RAO to a standard 5 Fr sheath in unselected patients undergoing transradial (TR) coronary angiography and/or intervention, and to assess the relative importance of sheath size and haemostasis protocol on the rate of RAO.
Methods and results: We conducted a randomised, multicentre, non-inferiority trial comparing the GSS6Fr against the standard GS5Fr in patients undergoing TR coronary angiography and/or intervention. Patients in each group were subsequently randomised to undergo patent haemostasis or the institutional haemostasis protocol. The primary endpoint was the occurrence of RAO at discharge. A total of 1,926 patients were randomised in 12 centres. The incidence of RAO was 3.47% with GSS6Fr compared with 1.74% with GS5Fr (risk difference 1.73%, 95% CI: 0.51-2.95%; pnon-inferiority=0.150). Patients randomised to patent haemostasis had a similar rate of RAO compared with institutional haemostasis (2.61% vs. 2.61%, p=1). There was no difference with regard to all secondary endpoints, including vascular access-site complications, local bleeding and spasm.
Conclusions: In this large multicentre randomised trial, the GSS6Fr was associated with a low event rate for the primary endpoint (RAO), although non-inferiority to the GS5Fr was not met, due to a lower than expected rate of RAO in the GS5Fr group. As compared to institutional haemostasis, the use of patent haemostasis was not associated with a reduced rate of RAO.
Abbreviations
ACS: acute coronary syndrome
Fr: French
GS5Fr: 5 Fr Glidesheath
GSS6Fr: 6 Fr Glidesheath Slender
OD: outer diameter
OR: odds ratio
PCI: percutaneous coronary intervention
RAO: radial artery occlusion
TF: transfemoral
TR: transradial
Introduction
Since the first transradial (TR) percutaneous coronary intervention (PCI) performed in 19921, numerous trials and meta-analyses have shown that TR access compared to transfemoral (TF) access is associated with fewer vascular and bleeding complications2-7, improved patient comfort8 and lower cost9. The recognition of these clinical benefits has led to a growing adoption of the radial artery as the primary access site for cardiac catheterisation throughout the world. One of the remaining limitations of radial access is related to the small size of the radial artery. Use of a sheath larger than the inner lumen of the radial artery (“sheath to artery mismatch”) promotes vascular injury and is a strong predictor of radial artery occlusion (RAO)10. Although the use of a 5 Fr sheath is associated with a lower rate of RAO than a 6 Fr sheath11-13, it only allows the use of small guiding catheters with limited back-up support and restricts the use of adjunctive devices in case of complex PCI. Besides sheath size, the absence of blood flow in the radial artery during radial haemostasis (“occlusive” haemostasis) is another important predictor of RAO, and the application of a patent haemostasis protocol has been demonstrated to be an effective strategy to prevent RAO14,15. The 6 Fr Glidesheath Slender® (GSS6Fr) (Terumo Corp., Tokyo, Japan) is a recently developed thin-walled radial sheath with an outer diameter (OD) that is smaller than the OD of standard 6 Fr sheaths and approaches the average OD of contemporary 5 Fr sheaths. This new sheath has the potential to minimise radial artery trauma and thus occlusion while allowing treatment of complex lesions using standard 6 Fr guiding catheters16. However, it is unclear whether the routine use of this new 6 Fr slender sheath will result in similar rates of RAO to a standard 5 Fr sheath in unselected patients undergoing TR coronary angiography and intervention. Therefore, we performed a large randomised, multicentre, single-blind, non-inferiority trial comparing the GSS6Fr against the standard 5 Fr sheath (GS5Fr) (Terumo) in patients undergoing TR coronary angiography and/or intervention. In order to assess the relative importance of sheath size and post-procedural haemostasis protocol on the rate of RAO, we carried out a factorial randomisation comparing a patent haemostasis protocol against the standard institutional haemostasis protocol.
Methods
STUDY DESIGN AND PARTICIPANTS
The Radial Artery Patency and Bleeding, Efficacy, Adverse evenT (RAP and BEAT) trial was an international multicentre, prospective, single-blind, randomised clinical trial using a 2×2 factorial design, assessing non-inferiority of the GSS6Fr against the GS5Fr, and superiority of patent haemostasis against institutional haemostasis in patients undergoing TR coronary angiography and/or intervention (ClinicalTrials.gov identifier: NCT02269449). This was an investigator-initiated trial and was sponsored by NPO International TRI Network. The trial was conducted in 12 centres in Japan, Europe and the USA. Patients were eligible for enrolment if they were to undergo TR coronary angiography and/or intervention. General exclusion criteria were as follows: 1) inability to puncture the radial artery, 2) presence of another medical illness that may cause non-compliance with the protocol or confound data interpretation, 3) haemodialysis patients, and 4) patients with acute coronary syndrome (ACS). The trial was approved by the institutional review board of each participating centre, and all patients gave written informed consent.
SHEATH DESCRIPTIONS
The GSS6Fr has been developed as a 6 Fr-compatible radial sheath with a hydrophilic coating and a thinner wall than current 6 Fr sheaths. The thickness of the sheath wall has been reduced from 0.20 to 0.12 mm, while maintaining an inner diameter of 2.22 mm. As a result, the OD has been reduced from 2.63 to 2.46 mm. The GS5Fr is a standard 5 Fr-compatible radial sheath with a hydrophilic coating and an OD of 2.29 mm (Figure 1).
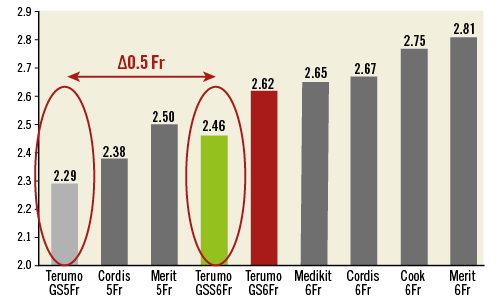
Figure 1. Comparison of sheath OD by manufacturer (with permission from Terumo).
RANDOMISATION
The trial had a 2×2 factorial design. After providing written informed consent, patients were centrally allocated (1:1) to receive the GSS6Fr or the GS5Fr. Patients in each group were immediately allocated again (1:1) to undergo patent haemostasis protocol or the standard institutional haemostasis protocol.
STUDY PROCEDURES
Radial artery puncture was performed using either an anterior wall puncture or counter-puncture as previously described17 and according to operator preference. Cardiac catheterisation and PCI technique were per operator preference. After successful sheath insertion, a vasolytic drug cocktail of calcium channel blockers and nitrates was given through the side port of the sheath in all patients. A minimal initial bolus of 5,000 IU unfractionated heparin was recommended in all patients. Diagnostic procedures were performed using 4 or 5 Fr catheters. In case of PCI, a 5 or 6 Fr guiding catheter was chosen according to patient allocation, operator preference and lesion complexity. However, the use of a 5 Fr guiding catheter was recommended in case of ad hoc PCI in patients assigned to GS5Fr. In case of upsizing to a larger sheath, the protocol mandated the use of a standard Glidesheath. An adjunctive bolus of heparin was given during PCI if needed in order to achieve an activated clotting time of 250-300 sec. Administration of glycoprotein IIb/IIIa inhibitors and/or bivalirudin was at the discretion of the operator. After completion of the TR procedure, the arterial sheath was removed and haemostasis was performed according to patient randomisation (patent haemostasis protocol vs. institutional haemostasis protocol).
A concise patent haemostasis protocol was provided to each centre before initiating the trial and all investigators received training on the protocol prior to enrolling subjects. For each centre, the patent haemostasis protocol included all of the following steps: 1) initial inflation of the TR Band (Terumo) at the site of radial puncture with 12 cc of air to facilitate sheath removal, 2) evaluation of radial artery patency by plethysmography during manual compression of the ipsilateral ulnar artery, and 3) in case of occlusive compression of the radial artery, gradual deflation of the TR Band until the plethysmographic signal returned, confirming radial artery patency. It was recommended to achieve the lowest pressure necessary to maintain haemostasis for each patient without bleeding. Institutional haemostasis protocols consisted mainly of the application of various haemostatic devices in order to achieve radial haemostasis under minimal pressure, followed by gradual decompression but without confirmation of radial patency. It was recommended to limit radial compression to a maximum of four hours for both diagnostic and PCI procedures. Of note, two centres were already using patent haemostasis as the institutional standard protocol. All cases were performed by experienced radial operators having an average annual volume of 250 TR-PCI.
ENDPOINTS AND DEFINITIONS
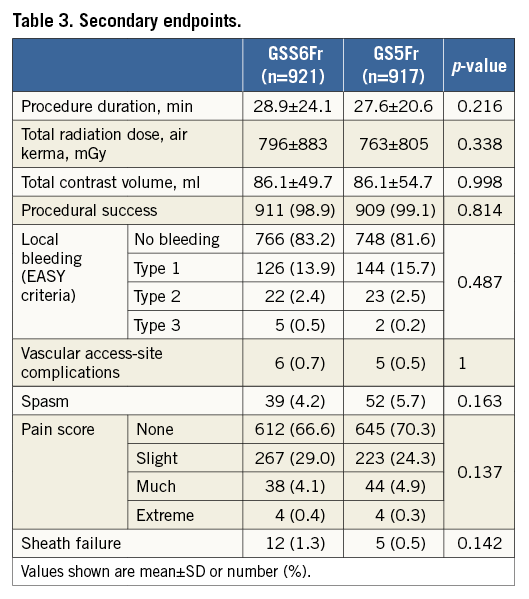
The primary endpoint was the rate of RAO at discharge. Secondary endpoints were procedural success, vascular access-site complications, local bleeding, radial spasm, total procedural time, total amount of contrast dye, total radiation dose, sheath failure and pain score. RAO was defined as the absence of a radial pulse assessed clinically together with the absence of flow assessed by Doppler ultrasound examination of the radial artery. The physician assessing radial patency was blinded to the assigned sheath. Procedural success was defined as completion of the planned procedure through the initially selected radial access route. Vascular access-site complication was defined as any documented vascular damage that included, but was not limited to, vessel perforation, arterial dissection, pseudoaneurysm, and local haematoma. Radial spasm was defined as an inability to manipulate the guidewire or catheter in a smooth and pain-free manner and also as an inability to remove the sheath in a similar way at the end of the procedure. The diagnosis of puncture-site bleeding was made by visual assessment before discharge and classified according to the EASY criteria (type I, ≤5 cm diameter; type II, ≤10 cm diameter; type III, >10 cm but not above the elbow; type IV, extending above the elbow; and type V, anywhere with ischaemic threat of the hand)7. Sheath failure was defined as any device malformation leading to vascular complication and/or procedural failure. Pain score denoted the patient’s assessment of pain during radial artery sheath removal (1=none, 2=slight, 3=much and 4=extreme). Successful haemostasis was noted when removal of the haemostatic device could be performed within six hours after completion of the procedure.
STATISTICAL ANALYSIS
The trial was powered for non-inferiority of the GSS6Fr against the GS5Fr on the primary endpoint (RAO). The sample size calculation was based on the assumption of a rate of RAO of 5% in the control group (GS5Fr)10. We selected a non-inferiority margin of 2.5 percentage points for the risk difference. We estimated that the assignment of 1,900 patients in a 1:1 ratio to the GSS6Fr group versus the GS5Fr group would provide a power of 80% to demonstrate non-inferiority, assuming a rate of RAO of 5.0% with both devices with a one-sided alpha level of 0.025. We used Pearson’s chi-square test or Fisher’s exact test to compare categorical variables and the t-test to compare continuous variables. Potential risk factors for post-procedural RAO were investigated first by univariate logistic regression. A multivariate logistic regression model with all significant variables was established to estimate odds ratios (ORs) inclusive of 95% confidence bounds. All statistical analyses were performed with SPSS, Version 20.0 (IBM Corp., Armonk, NY, USA).
Results
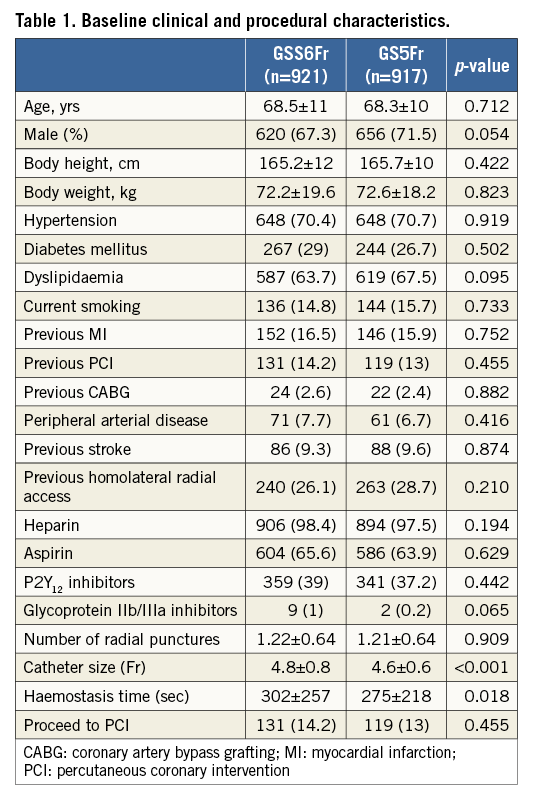
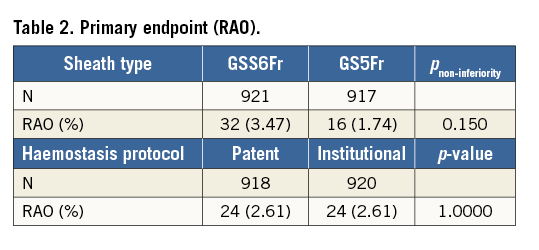
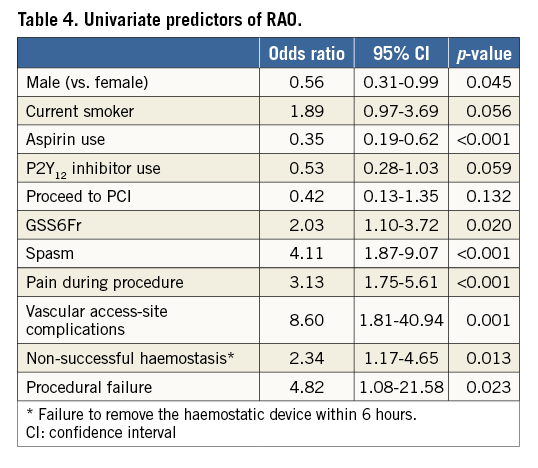
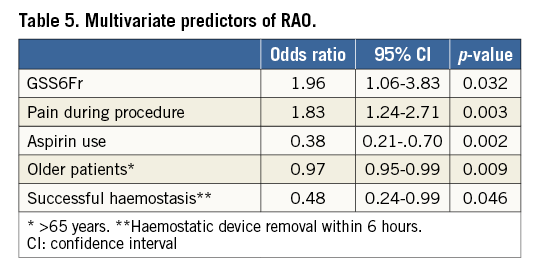
Between October 2014 and March 2016, a total of 1,926 patients were randomised at 12 sites in Japan, Europe and the USA. Eighty-eight patients were excluded for insufficient data (n=70) or failed radial puncture (n=18), leaving 1,838 patients for final analysis. Of these patients, 921 were assigned to GSS6Fr and 917 to GS5Fr. Of the GSS6Fr cohort, 448 and 473 were randomly assigned to patent or institutional haemostasis, respectively. Of the GS5Fr cohort, 470 and 447 were randomly assigned to patent or institutional haemostasis, respectively (Figure 2). The baseline characteristics of the study population are shown in Table 1. Previous access on the same radial artery was noted in 26.1% of patients assigned to GSS6Fr and in 28.7% of patients assigned to GS5Fr (p=0.210). In total, PCI was performed in 250 patients without difference between the two groups. Unfractionated heparin was used as the anticoagulant during diagnostic angiography and PCI for 98.4% and 97.5% in the GSS6Fr and GS5Fr groups, respectively. The mean used catheter size was significantly larger in patients assigned to GSS6Fr (4.8±0.8 Fr vs. 4.6±0.6 Fr, p<0.001). The haemostasis time was significantly longer with the GSS6Fr (302±257 vs. 275±218 sec, p=0.018), and significantly longer in Japanese vs. non-Japanese patients (378±253 vs. 159±136 sec, p<0.001). The study endpoints are shown in Table 2 and Table 3. The primary endpoint of RAO, as assessed by Doppler ultrasound imaging at discharge, occurred in 32 (3.47%) of 921 patients in the GSS6Fr group vs. 16 (1.73%) of 917 patients in the GS5Fr group. The upper bound of 2.95% for the risk difference was not lower than the pre-specified non-inferiority margin of 2.5%. Therefore, statistical non-inferiority was not achieved. Patients randomised to patent haemostasis protocol had a similar rate of RAO compared to institutional haemostasis protocol (2.61% vs. 2.61%, p=1), with no difference according to the sheath type (GSS6Fr group: 3.6% vs. 3.4%; GS5Fr group: 1.7% vs 1.8%, p=1 for both). In patients undergoing PCI (n=250), only three cases of RAO were noted (1.2%). Secondary endpoints of procedural success, vascular access-site complications, local bleeding, radial spasm, total procedural time, total amount of contrast dye, fluoroscopy time, sheath failure and pain score were not statistically different between the two groups. In patients assigned to GS5Fr, upsizing to a larger sheath was noted in 36 cases (3.93%) and did not impact on the rate of RAO (1/36 vs. 15/881, 2.78% vs. 1.7%; p=0.476). However, it was associated with a higher rate of radial artery spasm (11/36 vs. 41/881; 30.56% vs. 4.65%; p<0.0001). In patients assigned to GSS6Fr, use of a 6 Fr catheter was noted in 230 cases (25%). Although non-significant, the rate of sheath failure was numerically higher with the GSS6Fr (1.3% vs. 0.5%, p=0.142). Univariate and multivariate analysis of predictors of post-procedural RAO are presented in Table 4 and Table 5. The use of GSS6Fr, male sex, aspirin, spasm, pain during procedure, vascular complications, procedural failure and non-successful haemostasis were predictors of RAO in univariate analysis. In multivariate regression analysis, the use of GSS6Fr (OR: 1.96, 95% confidence interval [CI]: 1.06 to 3.83, p=0.032), pain during procedure (OR: 1.83, 95% CI: 1.24 to 2.72, p=0.003), age >65 years (OR: 0.97, 95% CI: 0.95 to 0.99, p=0.009), successful haemostasis (OR: 0.48, 95% CI: 0.24 to 0.99, p=0.046) and aspirin use (OR: 0.38, 95% CI: 0.21 to 0.70, p=0.002) were significantly associated with post-procedural RAO.
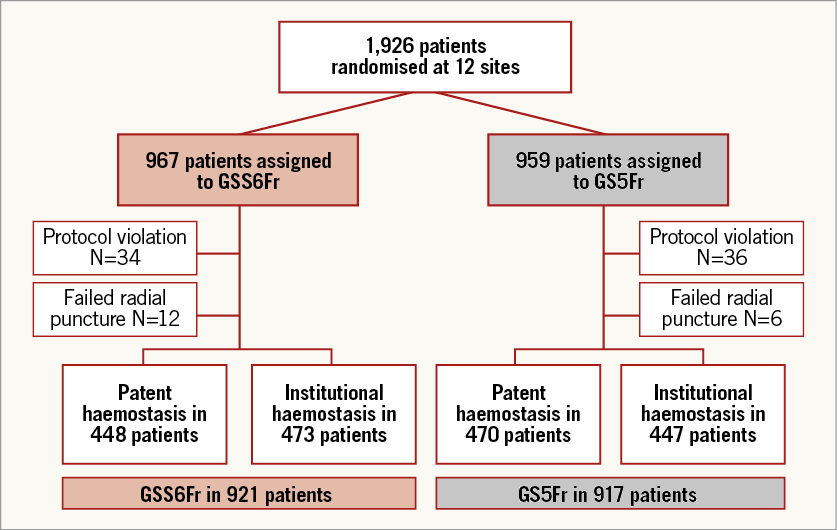
Figure 2. Study flow chart.
Discussion
Our study represents the largest intercontinental multicentre randomised trial comparing two different sheaths for TR access. It was also the first trial that aimed to assess the relative importance of sheath size and post-procedural haemostasis care on the rate of RAO. The major findings of the trial were: 1) the overall incidence of RAO for the entire study cohort was low (2.61%), reflecting outcomes in experienced radial centres all around the world; 2) our primary hypothesis that the new GSS6Fr would provide a similar rate of RAO to a conventional 5 Fr sheath could not be confirmed since statistical non-inferiority was not achieved; 3) there was no difference between the two groups with regard to all secondary endpoints, including vascular access-site complications, local bleeding and spasm; and 4) as compared to local institutional haemostasis protocols, the application of a patent haemostasis protocol did not reduce the rate of RAO.
TR access has become the default access site for coronary angiography and intervention in many countries, and strategies aiming at preserving the patency of the radial artery for future procedures are of the utmost importance18. Beyond baseline patient characteristics associated with an increased risk of RAO (i.e., low BMI, female patients), several procedural strategies have been shown to reduce the incidence of RAO, including reduction in sheath size, adequate anticoagulation and patent haemostasis10-16,19. The strong impact of sheath size on the rate of RAO is well known and related to the smaller size of the radial artery. Indeed, in two large studies using echo Doppler assessment of the radial artery, the proportion of patients with a mean radial diameter smaller than the minimal OD of a standard 6 Fr sheath ranged from 14% to 32% in men and from 27% to as high as 60% in women20,21. Therefore, the use of standard 6 Fr sheaths will induce a mismatch between the sheath and the radial artery in a significant proportion of patients, thereby increasing the incidence of severe radial flow reduction, spasm, radial injury, and ultimately acute or delayed RAO22. Although diagnostic coronary angiography and treatment of non-complex coronary lesions can be performed safely through 5 Fr sheaths and catheters, adjunctive devices such as rotational atherectomy or guide catheter extensions require the use of at least a 6 Fr guide catheter. Moreover, the treatment of bifurcation lesions often involves specific techniques (kissing balloon, two-stent techniques) that are only compatible with large-bore guide catheters. In order to overcome these anatomical limitations and minimise the risk of radial artery injury, radial operators and the industry have developed novel sheath-based and sheathless approaches23-26. The Glidesheath Slender represents the thinnest 6 Fr radial sheath currently available, with an OD close to the average diameter of standard 5 Fr sheaths. Preliminary data with this new sheath on 114 consecutive patients showed a high procedural success rate and low rate of vascular complications and RAO (0.88%)16. In the present large-scale randomised trial, we found a rate of RAO of 3.46% associated with this new sheath, which is one of the lowest reported so far for a 6 Fr-compatible sheath, if we compare it to the average rate of 11.6% for standard 6 Fr sheaths reported in a recent meta-analysis10. However, RAO rates in the 5 Fr group were significantly lower than expected, affecting the ability of the study to establish non-inferiority of the slender sheath for the primary outcome. This difference may in part be explained by the slight difference in the OD (GSS6Fr: 2.46 mm vs. GS5Fr: 2.28 mm), emphasising the impact of even a small increase in sheath OD (here 0.5 Fr difference) on the occurrence of RAO. It is important to note that only 14% of the study population underwent PCI, as many centres performed only diagnostic angiography followed by elective PCI, and one could argue against the routine use of a 6 Fr sheath in this setting. On the other hand, in centres performing mainly ad hoc PCI or with a large population of ACS patients, the GSS6Fr could represent an interesting alternative to a 5 Fr sheath, enabling performance of complex PCI without the need to upsize the sheath.
Although the overall rate of radial artery spasm did not differ between the two groups, upsizing to a larger sheath (mainly 6 Fr) was associated with a higher rate of radial artery spasm in the conventional 5 Fr group. The rate of sheath failure was numerically higher with the GSS6Fr and could be related to its thinner wall which may increase the risk of significant kinking. In this large trial, we also evaluated the impact of a systematic patent haemostasis protocol using the TR Band system vs. the standard institutional haemostasis protocol, by performing a second 1:1 randomisation. Interestingly, we found no additional benefit of using a patent haemostasis protocol on the rate of RAO. Of note, two centres were already using patent haemostasis as the institutional protocol (n=434 patients). Even when adding these patients to the patent haemostasis group in a per-protocol analysis, there was still no statistical difference between the two groups (2.28% vs. 3.14%, p=0.293), although the rate of RAO was numerically lower in the case of patent haemostasis. One can assume that the low rate of RAO observed in the two groups precluded any additional benefit of using a patent haemostasis protocol, reflecting that the trial was probably underpowered to detect a significant difference. It is also important to note that the benefit of patent haemostasis has been demonstrated at a time when the “standard haemostasis technique” was probably associated with a high rate of occlusive compression. Indeed, the instructions for use of the TR Band still recommend the inflation of 13 up to 18 cc of air which may lead to a substantial rate of occlusive compression. Due to the growing knowledge of the importance of non-occlusive compression, many centres have adapted their protocol by favouring the application of minimal pressure to obtain haemostasis together with a rapid deflation protocol. Interestingly, a recent study has demonstrated that, by using the TR Band system and a “rapid deflation technique” (minimal pressure inflation of up to 7 cc of air, 15 minutes after sheath removal), the vast majority of radial arteries (95%) disclosed patency, and this technique was associated with a significantly lower rate of RAO than a standard deflation technique (2% vs. 14.5%, p=0.005)27. Our findings therefore call into question the usefulness of a strict and systematic patent haemostasis protocol that can be time-consuming, and suggests that a “minimal pressure” strategy associated with early decompression of the haemostasis device may be an alternative approach that can result in a low incidence of RAO.
Study limitations
Despite being the largest multicentre randomised trial comparing two different sheaths for TR access, some limitations of the current trial need to be addressed. First, it was a single-blind trial and radial operators were aware of the randomised assignment. However, the outcome assessors for the primary endpoint (RAO) were blinded. Second, the low rate of PCI procedures in both groups may have precluded any benefit of using a 6 Fr sheath. Moreover, PCI procedures within the GS5Fr group were performed mostly with the use of 5 Fr guide catheters, reflecting lower PCI complexity not requiring large-bore guide catheters. However, PCI procedures may paradoxically have a protective effect against RAO since they are associated with more potent antiplatelet therapy and an increased level of procedural anticoagulation. The number of catheter exchanges in each group was not reported although it could have influenced the rate of radial artery trauma and occlusion. Third, the study design did not allow a comparison against a standard 6 Fr radial sheath. Future studies are therefore needed to compare the 6 Fr Glidesheath Slender against standard 6 Fr sheaths with a focus on vascular access-site complications and RAO. Finally, due to the unexpected low rate of RAO in the control group, our study was underpowered to demonstrate non-inferiority of the GSS6Fr over the GS5Fr, and the results should therefore be considered as inconclusive.
Conclusions
In this large multicentre randomised trial of patients undergoing TR access, the GSS6Fr was associated with a low event rate for the primary endpoint (RAO) although non-inferiority to the GS5Fr was not met, due to a lower than expected rate of RAO in the GS5Fr group. There was no significant difference between the two groups with regard to all secondary endpoints, including procedural success, vascular access-site complications, local bleeding and spasm. As compared to institutional haemostasis protocol, the use of a patent haemostasis protocol was not associated with a reduced rate of RAO as tested by these experienced radial centres.
| Impact on daily practice During TR access, the size of introducer sheaths and the haemostasis protocol have a strong impact on the rate of radial artery occlusion (RAO). Our large trial demonstrates that the use of the 6 Fr Glidesheath Slender is associated with one of the lowest reported rates of RAO (3.47%) for a 6 Fr-compatible sheath. However, non-inferiority of this new sheath against a standard 5 Fr sheath for the occurrence of RAO could not be demonstrated. Therefore, the choice of sheath size for TR access should be tailored according to the clinical indication by favouring the smallest sheath necessary to complete the procedure. During radial haemostasis, the application of a minimal pressure strategy and a rapid deflation technique may result in a low incidence of RAO. |
Conflict of interest statement
I. Gilchrist, S. Saito and S. Rao have received fees from Terumo Interventional Systems for educational talks and consulting. The other authors have no conflicts of interest to declare.

