Abstract
Aims: We assessed feasibility, efficacy and safety of a suture-mediated closure device, Perclose Proglide® (Abbott Vascular Devices, Santa Clara, CA, USA), for closure of the femoral vein access after percutaneous MitraClip® (Abbott Vascular Devices) implantation.
Methods and results: Venous access of 80 consecutive patients undergoing percutaneous mitral valve repair using the MitraClip device was managed either by manual compression, “figure eight” suture and compression bandage for 12 hours, or by applying the Proglide device for haemostasis after the procedure (40 patients each group). Patients with Proglide closure showed complete immediate haemostasis in 92.5% (37/40) and were immobilised with a compression bandage for only four hours. In the Proglide group, one arteriovenous fistula was observed and had to be treated by vascular surgery. The overall duration of stay on an intensive care unit was significantly reduced in the Proglide group (59.4±48.9 hours vs. 84.6±59.5 hours, p<0.005).
Conclusions: Using a suture-mediated closure device for the femoral vein after percutaneous MitraClip implantation is feasible and safe. This allows earlier patient mobilisation and may reduce post-interventional duration of stay on an intensive care unit.
Abbreviations
ACD: arteriotomy closure devices
ACT: activated clotting time
ASA: acetylsalicylic acid
AV: atrioventricular
BMI: body mass index
CRP: C-reactive protein
EF: ejection fraction
Fr: French
Hb: haemoglobin
Hct: haematocrit
ICU: intensive care unit
INR: international normalised ratio
MR: mitral regurgitation
MV: mitral valve
PMVR: percutaneous mitral valve repair
RBC: red blood cell concentrate
STS: Society of Thoracic Surgeons
TAVI: transcatheter aortic valve implantation
TEE: transoesophageal echocardiography
Introduction
Percutaneous edge-to-edge repair of mitral valves in patients with severe mitral regurgitation and high surgical risk has evolved as a suitable alternative to high-risk surgery. Currently, the most widely used device for percutaneous mitral valve repair (PMVR) is the MitraClip® system (Abbott Vascular Devices, Santa Clara, CA, USA)1-7. PMVR using the MitraClip device requires a large calibre sheath (24 Fr) introduced into the femoral vein. Haemostasis is usually achieved by manual compression which may be facilitated by a cutaneous figure-of-eight stitch8,9 followed by prolonged application of a compression bandage (usually ≥12 hours). This contributes significantly to patient discomfort and may trigger additional complications by immobilisation.
To date, no closure system has been certified for venous access sites. Arteriotomy closure devices (ACD)10 have been investigated extensively to reduce the risk of vascular complications following diagnostic coronary angiograms11,12, percutaneous coronary intervention procedures13-16, percutaneous aortic balloon valvuloplasty9,17, transcatheter aortic valve implantation (TAVI)18, endovascular aortic aneurysm repair19 and interventional radiological procedures20. The off-label application of these devices in procedures requiring large venous sheaths (e.g., atrial septal defect closure, patent foramen ovale closure, left atrial appendage device closure, percutaneous pulmonary valve implantation, patent ductus arteriosus closure, mitral valvuloplasty) has been described previously, but data are still limited21-26.
According to the manufacturer’s manual, the Perclose Proglide® device (Abbott Vascular Devices) is currently only certified for use in 5-21 Fr access sites (until April 2013 only 5-8 Fr) of the common femoral artery. For sheath sizes greater than 8 Fr, at least two devices and the preclosure technique24 are required.
In this study we investigated the feasibility, efficacy and safety of the Perclose Proglide device for the management of femoral vein access after percutaneous MitraClip implantation using the preclosure technique.
Methods
Between September 2009 and March 2013, 137 patients were treated with a MitraClip device at our institution. Of these patients, 80 who underwent MitraClip implantation between September 2011 and March 2013 were clearly outside the initial learning curve. In this group, 40 consecutive patients received femoral access-site closure by manual compression, “figure eight” suture and prolonged compression bandage for 12 hours. In the next 40 consecutive patients, haemostasis was tried by applying the Proglide device using the preclosure technique according to the manufacturer’s instructions for arterial access-site closure. In brief, after puncture of the femoral vein, a 7 Fr dilator was introduced over the wire, followed by the Proglide device. In contrast to an arterial access, intraluminal positioning of the Proglide device was confirmed by slow blood flow out of the side port (Figure 1). After positioning the suture using the preclosure technique, the Proglide device was removed and the vein was further predilated for guide insertion. All operators were familiar with the use of the Proglide device from numerous previous arterial applications. Following access-site closure, a light compression bandage was used for only four hours. Patient mobilisation was started as early as possible after removing the compression bandage. Figure 2 shows the different closure concepts on a model.
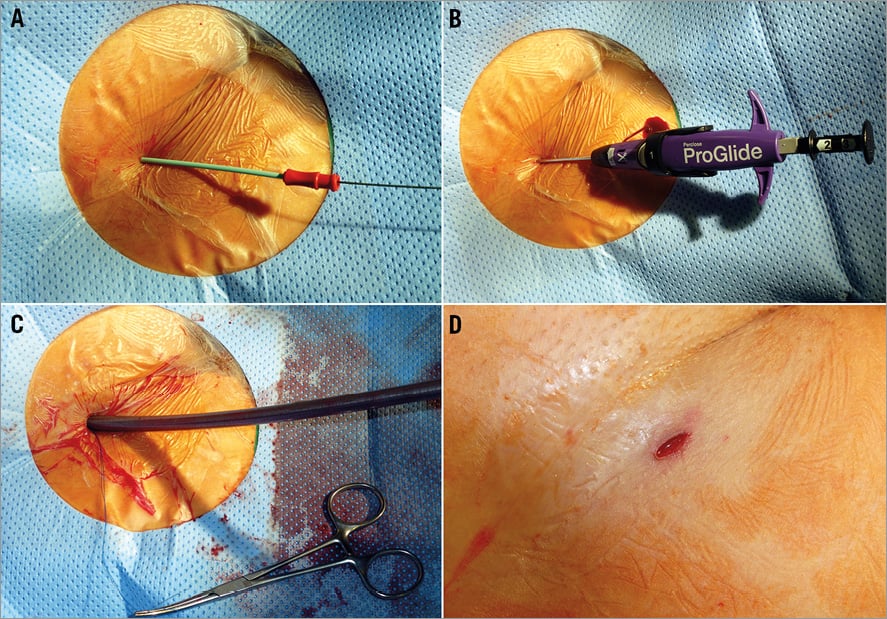
Figure 1. Closure of femoral vein access site with the Perclose Proglide® device applying the preclosure technique in the course of percutaneous edge-to-edge mitral valve repair. Following puncture of the femoral vein, a 7 Fr dilator is introduced over the wire. The Perclose Proglide device is inserted and the wire removed. Intraluminal positioning is confirmed by slow blood flow out of the side port. Preloaded sutures are safeguarded with a haemostat while a 24 Fr sheath for MitraClip delivery is inserted into the femoral vein. After sheath removal, the preformed surgical knot is slid down to the venous wall with a knot pusher for haemostasis and the suture is cut above the knot.
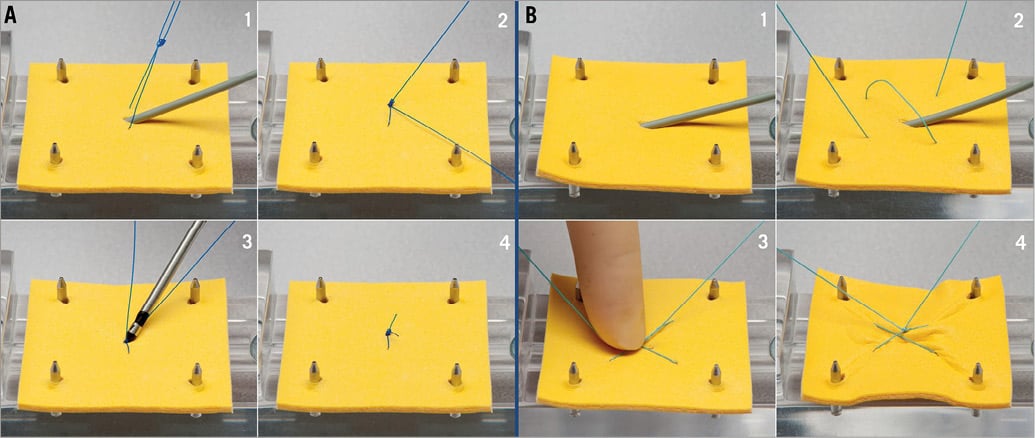
Figure 2. Models of different venous access site closure concepts. A) Model of Proglide closure on vein level. 1. The Proglide system has been prepared prior to the intervention in the preclosure technique. 2. At the end of the intervention, the sheath is removed and the rail suture limb is pulled simultaneously, thus advancing the knot. 3. The knot is further advanced on vein level with the knot pusher, followed by final locking of the knot. 4. At the end, the suture is cut with the knot pusher and the secured knot remains in the vessel wall. B) Model of “figure 8” suture on skin level. 1. & 2. While the sheath is still in the vein, a subcutaneous “figure 8” suture is prepared. 3. The sheath is removed and a knot is advanced to the skin simultaneously. 4. The knot is further tightened, in order to compress the skin superficially above the punctured vein.
Major vascular complications were defined as surgical repair of vascular injury, ultrasound-guided compression, groin-related transfusion or groin-related infection.
MitraClip implantation was performed under general anaesthesia and TEE control as published previously4,5. All patients received periprocedural heparin with a target ACT of 250-300 sec and prophylactic antibiotic therapy for three days after PMVR. Heparin was not routinely antagonised after the procedure.
All patients were transferred to our intensive care unit after the procedure (for at least 24 hrs). Patient mobilisation was started as early as possible. All patients were started on anticoagulation the day after the procedure.
The puncture site of all patients was examined clinically and by using colour-coded duplex sonography one day after the intervention.
All patients were informed about specific risks and alternatives and gave informed written consent to the procedure and pre- and post-interventional monitoring (data collection). The study protocol was in accordance with the local ethics committee.
Statistical analysis
Continuous variables were compared between groups using the Student’s t-test with mean±1 standard deviation reported. For non-continuous variables a Mann-Whitney U test was performed. Fisher’s exact test was applied to compare categorical variables with frequency and percent reported. For all tests, probability values of p<0.05 were considered statistically significant. For statistical analysis, GraphPad Prism® (GraphPad Software, Inc., La Jolla, CA, USA) as well as MedCalc (MedCalc Software, Ostend, Belgium) software was used.
Results
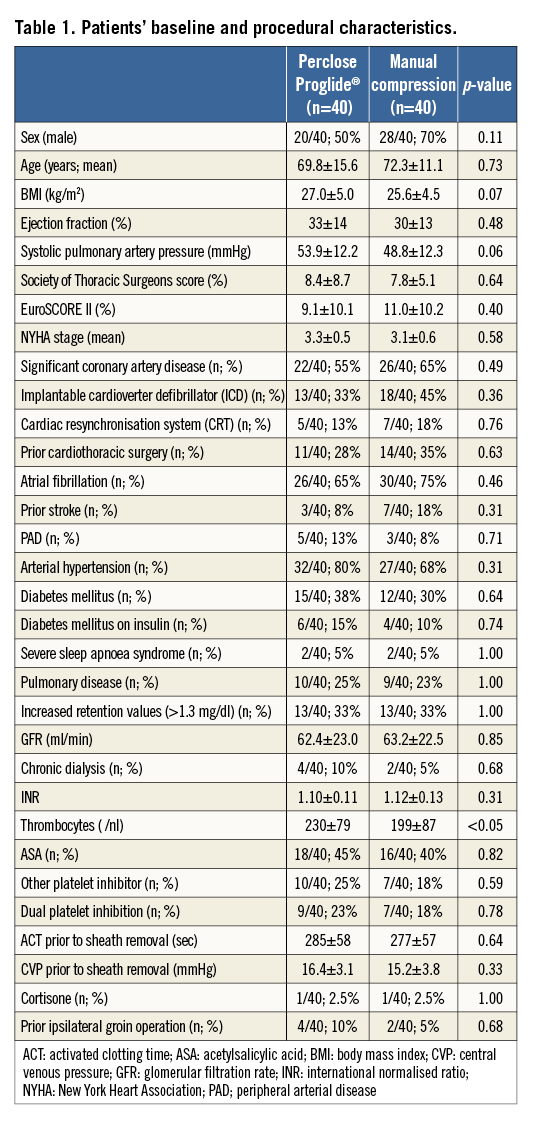
The predicted 30-day surgical perioperative mortality rate (STS score) in the two groups was similar (Proglide group 8.4±8.7% vs. manual compression group 7.8±5.1%; p=0.64). The EuroSCORE II did not show any significant differences either (Proglide group 9.1±10.1% vs. manual compression group 11.0±10.2%; p=0.40). There were no significant differences concerning baseline characteristics between the two groups. In particular, parameters possibly affecting access-site closure efficacy did not differ significantly between the Proglide group and the manual compression group (BMI 27.0±5.0 kg/m2 vs. 25.6±4.5 kg/m2, p=0.07; ACT prior to sheath removal 285±58 sec vs. 277±57 sec, p=0.64; central venous pressure prior to sheath removal 16.4±3.1 mmHg vs. 15.2±3.8 mmHg, p=0.33; INR 1.10±0.11 vs. 1.12±0.13, p=0.31; ASA 18/40 [45%] vs. 16/40 [40%], p=0.82; other platelet inhibitors 10/40 [25%] vs. 7/40 [18%], p=0.59; dual platelet inhibition 9/40 [23%] vs. 7/40 [18%], p=0.78; prior groin operation 4/40 [10%] vs. 2/40 [5%], p=0.68; chronic dialysis 4/40 [10%] vs. 2/40 [5%], p=0.68; current cortisone treatment 1/40 [2.5%] vs. 1/40 [2.5%], p=1.00). Only thrombocyte count showed a significant but clinically irrelevant difference between the Proglide group (230±79/nl) and the manual compression group (199±87/nl; p<0.05). A detailed overview of patients’ demographic characteristics is shown in Table 1.
In 37/40 patients (92.5%) receiving venous access-site closure with the Proglide device, immediate haemostasis (within ≤2 minutes) was achieved. In one case the closure device failed to deploy appropriately due to the operator’s inability to advance the knot to the venous surface as a result of the patient’s obesity (BMI 40 kg/m2). In another case the suture was accidentally cut. In these patients manual compression and “figure eight” suture were applied for access-site closure followed by a compression bandage for 12 hours. Deployment failure did not result in access-site complications. The only major vascular complication in the whole study was an AV fistula in one patient of the Proglide group, requiring surgical repair. It is unlikely that the AV fistula was a result of the Proglide placement.
In the manual compression group, immediate haemostasis was not evaluated, since even a complete closure of the puncture site with the “figure eight” suture would not rule out subcutaneous bleeding. In 4/40 patients (10%) of the manual compression group, protamine was used to antagonise heparin due to excessive ACT prolongation prior to sheath removal or TEE-associated oropharyngeal bleeding. In contrast, only 1/40 patients (2.5%) of the Proglide group received protamine treatment (p=0.36).
During hospitalisation one patient of each group experienced bleeding at the access site (1/40; 2.5%) requiring additional manual compression (evening after the procedure) without the need of blood transfusion.
Groin haematomas ≥6 cm occurred in one patient of each group (1/40; 2.5%). Groin haematomas >2 cm and <6 cm occurred in one patient of the Proglide group (1/40; 2.5%) and in three patients of the manual compression group (3/40; 7.5%; p=0.62). None of the affected patients in either group required a new compression bandage, prolonged hospital stay or transfusion. On follow-up examination all groin haematomas had regressed completely.
Pseudoaneurysm formation, venous stenosis, thrombosis, retroperitoneal bleeding or access-site infection was not detected in any of the patients of either group. Furthermore, there was no statistically significant difference of postinterventional increase of inflammation markers among patients treated with the Proglide device and traditional access-site closure (Proglide group: CRP before intervention: 13.6±22.9 mg/l, CRP two days after intervention: 28.2±31.3 mg/l, CRP max: 51.3±58.7 mg/l, leucocytes before intervention: 7.8±2.5/nl, leucocytes one day after intervention: 8.5±2.6/nl, leucocytes max: 10.9±5.2/nl; manual compression group: CRP before intervention: 7.2±9.7 mg/l [p=0.13], CRP two days after intervention: 27.7±21.3 mg/l [p=0.32], CRP max: 49.3±53.4 mg/l [p=0.92], leucocytes before intervention: 7.7±2.1/nl [p=0.87], leucocytes two days after intervention: 8.5±3.1/nl [p=0.80], leucocytes max: 10.9±4.8/nl [p=0.85]).
There was no statistically significant difference in haemoglobin (Hb) level drop four hours and one day after the procedure between the two groups (Proglide group: preprocedure Hb: 11.6±1.9 mg/dl [Hct=36%], Hb after four hours: 11.1±1.7 mg/dl [Hct=34%], Hb after one day: 10.9±1.7 mg/dl [Hct=33%]; manual compression group: preprocedure Hb: 12.1±1.4 mg/dl [Hct=36%], Hb after four hours: 11.9±1.5 mg/dl [Hct=36%], Hb after one day: 11.2±1.4 mg/dl [Hct=34%)]; p=0.08 [δ Hb four hours], p=0.87 [δ Hb one day], p=0.08 [δ Hct four hours], p=0.76 [δ Hct one day]).
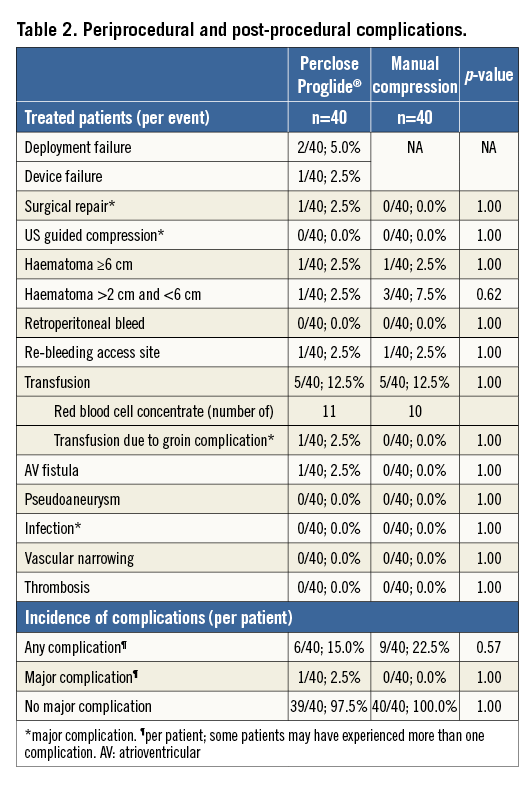
Red blood cell concentrate (RBC) transfusion due to access-site complications was required in one of the patients of the Proglide group (n=2 RBCs in the course of vascular surgery for the AV fistula) and in none of the patients of the manual compression group (p=1.00). In total, five patients from each group (5/40; 12.5%) received RBC transfusion within patients’ hospital stay, mainly due to reasons not related to access-site complications: Mallory-Weiss bleeding (n=1), oropharyngeal bleeding most likely due to intubation or TEE (n=3), perioperative bleeding during mitral valve replacement (n=3), pre-existing anaemia (Hb <8.0 mg/dl; n=2), AV fistula (as described above, n=1). Overall, in the Proglide group 11 RBC units were applied, whereas 10 RBC units were administered in the manual compression group. An overview of all observed complications is provided in Table 2.
In the Proglide group patient mobilisation could be started as early as four hours after PMVR, resulting in significantly shorter ICU length of stay compared to the manual compression group (59.4±48.9 hours vs. 84.6±59.5 hours; p<0.005). Physicians and nurses working on ICU were unaware of this study and based their decisions for transfer to a normal ward independently.
Discussion
In the course of cardiac catheterisation procedures groin complications remain the most common cause of morbidity27. Although there has been a reduction in adverse events with the use of increasingly smaller arterial sheaths28 and the more common use of arteriotomy closure devices (ACD)29,30, groin complications still occur in approximately 1% of cases.
The American Heart Association currently recommends the use of arteriotomy closure devices (ACD) after invasive cardiovascular procedures performed via the femoral artery to achieve faster haemostasis and allow earlier patient mobilisation31.
Suture-mediated ACDs have already become the main tool for arterial access closure after procedures that require sheath sizes up to 24 Fr32,33.
Recently, there has been an increase in the use of larger-sized venous sheaths, especially promoted by the introduction of PMVR using the MitraClip device, but also due to increasing numbers of percutaneous pulmonary valve implantation, atrial septal defect, patent foramen ovale and left atrial appendage closure. Even though the risk of vascular complications is inherently low with venous access compared to arterial access, using sheaths of these dimensions affects complication rates and early mobilisation. To date, only very few studies reporting on venous applications of ACDs have been published21-26. In the majority of these publications the Perclose device has been investigated. Only one of these reports the use of sheath sizes >14 Fr (22 Fr [n=15] and 24 Fr [n=1] for percutaneous pulmonary valve implantation)21.
Shaw et al were first to describe the use of a suture-mediated closure device (6 Fr Perclose Closer®, and 8 Fr Prostar® XL; both Abbott Vascular Devices) in patients who had venous access as part of their procedure23. A total of 42 patients were studied. In the majority, 7 Fr sheaths (up to 14 Fr) were used. Furthermore, Mylonas et al reported the use of the 6 Fr Perclose device for venous access-site closure (14 Fr) in a series of 45 patients undergoing antegrade aortic valvuloplasty24. No relevant complications occurred in this study.
Moreover, Ozawa et al demonstrated feasibility of venous access-site closure using the Perclose device in 14 of 20 children (70% success rate) undergoing percutaneous catheter interventions25. Time to haemostasis was significantly shortened compared to a control group. Larger study cohorts have been investigated by Mahadevan et al (146 patients with 205 femoral venous access sites) and by Hamid et al (243 patients with 310 venous access-site closures) more recently21,22. Complete haemostasis was achieved in 99% and 98%, respectively. In the study of Mahadevan et al, sheath sizes ranged from 7 to 14 Fr. In three cases a device failure occurred. Furthermore, in two patients major complications were observed. In the study of Hamid et al, mean venous access-site sheath diameter was 11.5 (±3) Fr (up to 24 Fr in one patient). In eight access sites the device failed to deploy. No major complications occurred.
In our study, we assessed feasibility, efficacy and safety of the Perclose Proglide device for the management of 24 Fr femoral vein access after percutaneous MitraClip implantation. As described before in arterial applications, handling of the closure device was very simple, providing appropriate vessel preclosure within a few minutes.
Thus, in 92.5% (37/40) of the patients, successful femoral vein access-site closure was achieved by applying the Proglide device. The three deployment failures, resulting from the patient’s obesity or accidental suture cut, were not followed by any access-site complications. Comparable device deployment difficulties have been described before21 and obesity has been associated with an increase in arterial access-site-related complications in various studies32,34-36.
Immediate haemostasis was achieved in all 37 patients with successful deployment of the Proglide device. This is comparable to success rates reported in other studies using suture-mediated devices for femoral vein access closure; however, sheath sizes investigated in these trials were significantly smaller as described above21-24,26.
There was no significant difference in overall incidence of complications among patients treated with the Proglide device and manual compression, respectively. Among patients receiving device-mediated access-site closure, one major complication occurred: an 80-year-old male experienced AV fistula formation requiring surgical repair and perioperative red blood cell concentrate transfusion. However, preprocedural Hb level was already low (8.6 g/dl) and PMVR was performed as a bail-out strategy in this critically ill patient37 (STS score 48.90%; EuroSCORE II 38.75%) suffering from ischaemic cardiomyopathy with severely decreased left ventricular function (EF 30%) and terminal renal failure with chronic dialysis. Furthermore, this patient had experienced prior ipsilateral groin operation one month before PMVR for an inguinal hernia repair. It is unlikely that AV fistula formation was related to the use of the Proglide device.
While a systematic follow-up of all patients was not possible, 60% of all treated patients have been seen during a six-month follow-up visit, which always includes assessment of further vascular complications (such as deep vein thrombosis) or complaints (such as painful groins). None of the patients has ever reported problems after the interventional hospitalisation, independent of the closure technique.
In patients receiving access-site closure with the Proglide device, mobilisation could already be started four hours after PMVR, resulting in a significantly reduced time to transfer to a regular ward. This may contribute to fewer midterm complications, faster recovery and more economic use of resources.
Limitations
This study is limited by the lack of a randomised design. Thus, in particular, a further advance in the operator learning curve and experience of the ICU staff may have affected the results. We tried to minimise this effect by starting this analysis after the first 57 cases. Also, the ICU staff were able to decide independently about mobilisation and were not aware of the study. The operators had previous experience with both arterial and venous Proglide closures (about 50 arterial closures for medium and large arterial sheaths up to 20 Fr and about 15 venous closures in patients with mitral interventions). Thus, a device-related learning curve was minimised.
The lack of a randomised design particularly limits the comparison of the significantly different duration of intensive care unit time. For a more thorough and valid analysis of benefits of the suture-based closure device, a future randomised trial will be needed. This proof of principle study may justify a larger randomised trial.
Conclusion
Off-label preclosure of large calibre venous access sites (24 Fr) with the Perclose Proglide device in MitraClip procedures allows rapid and safe haemostasis, thereby improving patient comfort, allowing early mobilisation and reducing patients’ duration of post-intervention stay on an ICU.
| Impact on daily practice Haemostasis of large calibre venous access sites (up to 24 Fr, e.g., in the course of percutaneous mitral valve repair employing the MitraClip device) is usually achieved by manual compression that may be facilitated by a cutaneous figure-of-eight stitch followed by prolonged application of a compression bandage. This contributes significantly to patient discomfort and may trigger additional complications by immobilisation. The off-label application of a suture-mediated closure device (Perclose Proglide®) for access-site closure in procedures requiring large venous sheaths allows rapid and safe haemostasis, thereby improving patient comfort, allowing early mobilisation and reducing patients’ duration of stay on an ICU postinterventionally. |
Conflict of interest statement
The authors have no conflicts of interest to declare.

