
Abstract
Aims: Iatrogenic pseudoaneurysms of the femoral artery lead to increased morbidity and mortality, especially when surgical treatment is necessary. Manual compression and thrombin injection are commonly used to occlude the pseudoaneurysms. However, in some cases these treatment options are inapplicable or unsuccessful. The aim of the present study was to examine the feasibility, effectiveness and safety of a novel approach with the use of suture-based closure devices to treat pseudoaneurysms.
Methods and results: Between January 2014 and May 2016, a total of eight iatrogenic pseudoaneurysms of the femoral artery were treated by the interventional closure technique after at least one ineffective attempt at manual compression. After puncture of the cavity, a PTCA guidewire was used to pass the neck of the pseudoaneurysm and a sheath was inserted in the femoral artery. Afterwards, a suture-based closure system (ProGlide) was used to occlude the neck. All eight pseudoaneurysms were successfully obliterated. No complications occurred during the procedure.
Conclusions: The new interventional technique presented in this study fills the gap in successfully treating pseudoaneurysms that cannot be obturated with conventional techniques. By implementing this new technique in clinical practice, a significant number of open surgical repairs could be prevented.
Abbreviations
CFA: common femoral artery
CTO: chronic total occlusions
EVAR: endovascular aneurysm repair
MP: multipurpose catheter
PA: pseudoaneurysm
PFA: profunda femoris artery
PTCA: percutaneous transluminal coronary angioplasty
SFA: superficial femoral artery
TAVI: transcatheter aortic valve implantation
Introduction
Iatrogenic pseudoaneurysms of the femoral artery are still common and lead to increased morbidity and mortality. Despite the widespread use of the radial approach in coronary angiography with a reduction of puncture-site complications, the increasing numbers of novel interventional procedures such as transcatheter aortic valve implantation (TAVI), endovascular aneurysm repair (EVAR), reopening of chronic total occlusions (CTO), rotablation or complex electrophysiological studies have led to the persistence of the femoral approach due to the use of larger sheath sizes. Most procedures require additional anticoagulation or antiplatelet therapy, which further increases the risk of vascular complications.
The incidence of iatrogenic pseudoaneurysms of the femoral artery ranges from 0.2% to 2.9% in diagnostics, up to 8% in interventional procedures1-4. There are many procedure-related and patient-related risk factors, for example gender, age, body mass index as well as puncture site, sheath size, emergency procedures and concomitant anticoagulation and antiplatelet therapy, which lead to an increased incidence of femoral pseudoaneurysms5,6.
The most common non-surgical treatment option in the case of post-interventional formation of pseudoaneurysms is manual compression, which is often insufficient and painful for the patient.
The main intention is to avoid pseudoaneurysms, as these will lead to increased hospital stays and mortality, especially when surgical treatment is necessary7,8.
Therefore, an alternative to open surgical repair seems reasonable to treat pseudoaneurysms. Until now, the most common treatment has been the injection of thrombin, which leads to complete thrombosis in 80% to 100% of cases9,10. Unfortunately, complications such as intra-arterial thrombosis or anaphylactic reactions can occur11,12. In particular, a very large cavity or a wide neck of the pseudoaneurysm is considered as an exclusion criterion for thrombin injection. Hence, alternative strategies to close the pseudoaneurysm in some patients are desirable.
With the introduction of vascular closure devices, it has become possible to close even larger access sites interventionally, as the period to complete haemostasis, complication rates and short-term quality of life have improved so that these devices are used in many interventional procedures13-15.
The aim of the present study was to examine the feasibility, effectiveness and safety of a novel approach with the use of suture-based closure devices to treat pseudoaneurysms.
Material and methods
Between January 2014 and May 2016, a total of eight iatrogenic pseudoaneurysms of the common or superficial femoral artery were treated by the interventional closure technique. The diagnosis was revealed by ultrasound. Furthermore, the different anatomic structures of the femoral artery, neck and cavity of the pseudoaneurysm (PA) were visualised sonographically. There was at least one attempt at ultrasound-guided compression for at least 30 minutes to achieve complete thrombosis. All patients gave written consent to interventional closure of the PA.
The crucial point of our procedure is to get a wire from the femoral artery, through the neck of the PA and out of the patient. After reaching that point, a suture-based closure device could be used to close the blood supply of the PA. In the following, we describe the several steps in detail. After local anaesthesia, the cavity of the PA was punctured guided by ultrasound and a guidewire (Emerald® Diagnostic Guidewire, 0.035-inch, 150 cm; Cordis, Cardinal Health, Milpitas, CA, USA) was positioned in the cavity. A 5 Fr sheath was inserted over this wire in the direction of the neck. Afterwards, a 4 Fr catheter (Multipurpose or Judkins Right; Cordis) was used to facilitate steering of the percutaneous transluminal coronary angioplasty (PTCA) guidewires then used. In some cases, a 4 Fr sheath was first inserted depending on the anatomy of the aneurysm in order to visualise the cavity with contrast medium and a second 5 Fr sheath was introduced after a vertical puncture to the neck of the aneurysm (Figure 1, Figure 2).
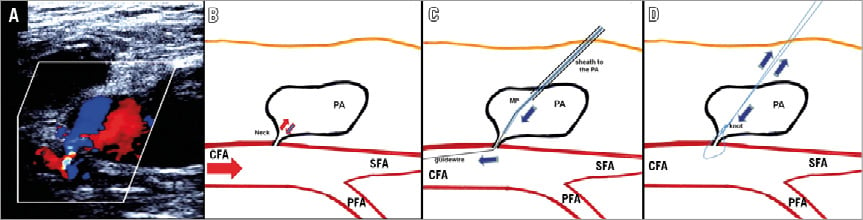
Figure 1. Schematic illustration: the interventional treatment of a pseudoaneurysm by a suture-mediated closure system. A) Large pseudoaneurysm on colour Doppler. B) Typical blood flow patterns (inflow and outflow) within the neck of a pseudoaneurysm. C) After placement of a sheath into the PA (in the direction of the neck), passage of the guidewire through the neck of the pseudoaneurysm in the common femoral artery directed with a Multipurpose catheter (MP) is performed. D) By getting a wire into the CFA, the occlusion of the neck by the Suture-Mediated Closure System, ProGlide, can be performed in the same manner as described in the instruction manual. After the closure system is withdrawn, a knot is tied in the suture and pushed down to the neck for haemostasis. CFA: common femoral artery; MP: Multipurpose catheter; PA: pseudoaneurysm; PFA: profunda femoris artery; SFA: superficial femoral artery
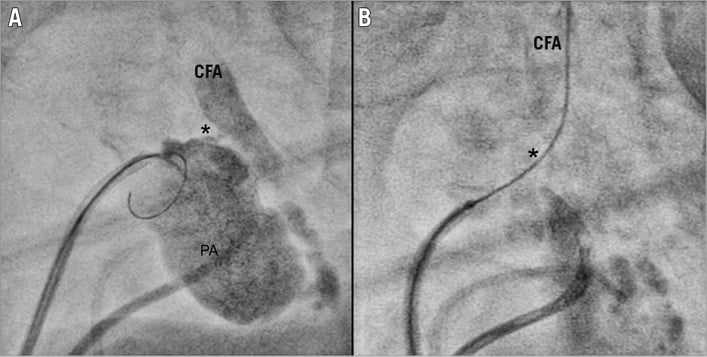
Figure 2. Process of occluding the pseudoaneurysm visualised by X-ray-based imaging. A) Visualisation of the pseudoaneurysm by contrast medium via a second sheath and the guiding of the wire with the aim of passing the neck. B) Achievement of the passage of the wire through the neck into the common femoral artery. * location of the neck. CFA: common femoral artery
Different PTCA guidewires (e.g., PILOT® 50, 0.014-inch, 190 cm; Abbott Vascular, Santa Clara, CA, USA) were used for passing through the neck into the femoral artery directed with a fluoroscopy-guided 4 Fr Multipurpose catheter. Afterwards, the 4 Fr catheter was advanced through the neck into the femoral artery. The correct intravascular position of the catheter was confirmed by angiography. Next, a 0.035-inch guidewire (Emerald Diagnostic Guidewire, 0.035-inch, 150 cm; Cordis) was placed through the catheter into the femoral artery and a Perclose ProGlide® 6F Suture-Mediated Closure (SMC) System (Abbott Vascular) was used to occlude the neck of the pseudoaneurysm. Total closure of the neck, loss of flow and complete thrombosis of the cavity were confirmed 15 minutes later by ultrasound (Figure 1). Moreover, an accidental compromise of the femoral artery blood flow was ruled out sonographically. All interventional procedures were performed without disruption to antiplatelet therapy or anticoagulation.
Success rates, complications, procedure time and fluoroscopy time were recorded. Further baseline characteristics of the patients such as age, sex, concomitant pre-/peri-interventional anticoagulation, body mass index (BMI) and initial type of intervention were registered.
Results
Baseline characteristics are shown in Table 1. Average age was 78.6±6.6 years. Three of the eight patients were male, mean BMI was 26.2±4.2 kg/m2, and five of the eight patients had a history of hypertension and diabetes mellitus. All patients received concomitant anticoagulation or platelet inhibitor therapy. In six of the eight patients, the initial procedure leading to pseudoaneurysm was coronary angiography or percutaneous coronary intervention, while one patient underwent TAVI and one underwent left atrial appendage occlusion. Puncture site localisation was the common femoral artery in six patients and the superficial femoral artery in two patients.
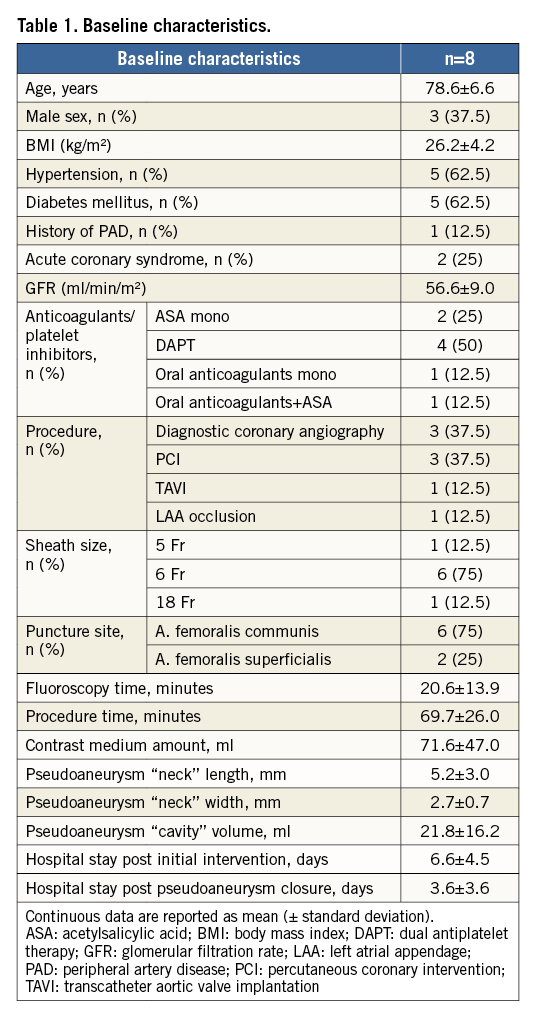
Mean fluoroscopy time was 20.6±13.9 minutes, mean contrast medium amount was 71±47.0 ml, and mean procedure time was 69.7±26.0 minutes. The procedure time of the first four patients tended to be longer than that of the last four patients (80.8±13.2 vs. 58.9±33.0 minutes, p=0.26), which could suggest the existence of a learning curve.
The mean length and width of the “neck” of the PA were 5.2±3.0 mm and 2.7±0.7 mm, respectively. Hospital stay after the initial procedure was 6.6±4.5 days, and 3.6±3.6 days after the interventional closure procedure. Five of the eight patients were able to be discharged the following day. Three of the eight patients suffered from a heart failure and so their stay in the hospital was extended, primarily due to heart failure treatment and not because of the PA closure.
In all cases, a ProGlide system was used to occlude the PA and success rates were 100%. There were no complications such as arterial thrombosis or occlusion with the use of the ProGlide system.
Discussion
With increasing age and concomitant diseases, complication rates in vascular interventions also increase. Surgical interventions for femoral pseudoaneurysms should be avoided in order to reduce hospital stay and mortality. The appearance of lymphatic fistulas, the need for sore draining and prolonged wound healing are common after groin surgery8. The concomitant use of oral anticoagulants in particular increases the bleeding rates.
With stringent use of the radial approach, the correct puncture site of the common femoral artery and accurate compression after the procedure, the number of post-interventional pseudoaneurysms has been reduced, but cannot be completely avoided. The complexity of some interventions still requires a femoral approach due to the need for larger sheath sizes, even under concomitant therapy with oral anticoagulants or antiplatelet therapy.
Thrombin injection is commonly used for closing pseudoaneurysms, but the application of thrombin is hazardous in some cases and might cause complications16. In contrast to pseudoaneurysms with long “necks”, which are suitable for compression or thrombin injection, a short and wide “neck” should be treated with this new technique instead. Therefore, different closure techniques should be considered depending on the anatomy of the PA.
With results from the current study, a new and innovative interventional therapeutic option for treating incompressible post-interventional pseudoaneurysms has been established. The salient findings of the study are that the presented technique to close femoral pseudoaneurysms is effective in 100% of cases, even though a prior attempt by ultrasound-guided compression failed. Furthermore, this technique is safe, and without any severe complications.
Another important finding of this study is the low complication rate of the interventional closure technique. The fact that there is only a puncture of the pseudoaneurysm and no further arterial puncture minimises additional complications. Other techniques such as balloon inflation or implantation of a covered stent in the femoral artery to interrupt blood flow within the PA require a second arterial puncture and should only be used as a bail-out intervention in haemorrhagic shock.
This new technique has many advantages. First, patients with ongoing oral anticoagulation in particular can be successfully treated, as a suture-based closure is effective independent of concomitant medications. Second, pseudoaneurysms even with wide and short necks, which are not compressible, can be treated. Third, potential complications which might occur with the use of thrombin injection such as peripheral embolism of thrombin or anaphylactic reactions are impossible.
Nevertheless, there are still aspects of this new technique that have to be improved. First, a mean procedure time of 69 minutes and a mean fluoroscopy time of 20 minutes seem quite long. The most time-consuming and difficult part is the placing of the guidewire through the neck of the PA into the femoral artery. Especially in long and tortuous necks, this is often associated with a prolonged procedure time. Perhaps 3D ultrasound guiding could simplify this step and reduce the fluoroscopy time. Furthermore, this could protect the physician from radiation, as work under fluoroscopy is necessary in many steps of the procedure. However, the use of ultrasound to visualise the puncture site and wire passage might be difficult, especially when a massive haematoma is present.
Moreover, profound experience in the use of suture-based vascular closure devices is essential for procedural success and avoidance of injury to the native vessels. Relative contraindications for this procedure are, as mentioned above, a long and tortuous neck of the PA, whereby these patients might benefit from a thrombin injection. On the other hand, a broad neck of the PA might not be sufficiently covered and closed by the ProGlide system. In that case, the procedure might be performed in combination with another closure device such as the Angio-Seal™ (St. Jude Medical, St. Paul, MN, USA). Absolute contraindications for the procedure are a pseudoaneurysm near the bifurcation of two vessels, since such a puncture site might result in an intimal dissection or an acute vessel closure.
Limitations
A larger, multicentre, randomised study would be beneficial, comparing the presented interventional approach and conventional therapies, for example surgical management or thrombin injection. It could provide some valuable information about its efficacy compared with the conventional therapies, also helping to identify patients with favourable or unfavourable characteristics for this percutaneous approach.
Conclusions
The new interventional technique presented in this study fills a gap by successfully treating pseudoaneurysms that cannot be obturated with conventional techniques. With this new technique implemented into clinical routine, the number of surgical interventions can be further reduced.
| Impact on daily practice Iatrogenic pseudoaneurysms of the femoral artery are still common and lead to increased morbidity and mortality. With the introduction of this new technique even those pseudoaneurysms that are not feasible for thrombin injection or sonography-guided compression could be closed sufficiently without any severe complications. Therefore, a great number of surgical procedures with increased hospital stay and morbidity could be prevented. |
Conflict of interest statement
The authors have no conflicts of interest to declare.

