Abstract
Aims: To evaluate efficacy of percutaneous ultrasound-guided thrombin injection (UGTI) of iatrogenic femoral artery pseudoaneurysm (PSA) and to identify the risk factors associated with PSA recurrence.
Methods and results: We treated 140 patients aged 76 years (range 49-83) presented with femoral artery PSA after cardiac catheterisation by percutaneous UGTI (500 IU/ml solution of activated human thrombin). Factors associated with the recurrence of PSA were analysed. One hundred nineteen patients were successfully treated by one injection of thrombin (immediate success rate 85%). In 19 patients (13.6%), short local compression following injection was needed for complete occlusion (overall success rate 98.6%, 138/140). In one case, progression of PSA required conversion to surgery (0.7%). In one patient with pre-existing stenosis of superficial femoral artery, acute limb ischaemia developed after UGTI (0.7%). The recurrence of PSA in 30-days follow-up (10 patients, 7%) was associated with obesity (BMI>30, OR=1.39, 95% CI 1.09-1.78, p<0.05), and with extensive combination of anti-aggregation and anti-coagulation therapy (OR=2.11, 95% CI 1.23-3.62, p<0.0001) as revealed by both univariate and multivariate analysis.
Conclusions: The UGTI is a safe and effective treatment of iatrogenic femoral artery PSA. Recurrence is low and associated with obesity and extensive use of combined anti-aggregation and anti-coagulation therapy.
Introduction
The incidence of iatrogenic femoral artery pseudoaneurysm (PSA) after cardiac catheterisation ranges from 0.3% to 2.0% and has increased over recent years with intensive anti-aggregation therapy and as a result of the use of larger-size catheters for interventional procedures1-5. It should be noted that even the use of closure devices didn’t decrease the incidence as compared to conventional compressive management5-7.
Ultrasound guided thrombin injection (UGTI) is rapid, minimally invasive and a highly successful treatment of post-catheterisation PSA8,9. However, the predictors of the failure of thrombin occlusion, and recurrence of PSA after thrombin application have not been evaluated. Accordingly, the aim of our study was to evaluate the efficacy of this technique and to assess the risk factors associated with recurrence of femoral PSA after occlusion by thrombin injection.
Methods
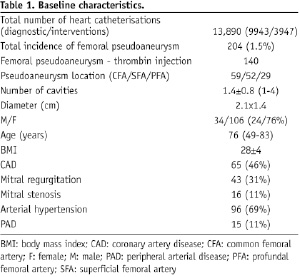
From February 2002 to December 2006, 140 patients (male/female: 34/106) aged 76 years (range 49-83) who presented with PSA after cardiac catheterisation at the site of the femoral artery were treated by percutaneous UGTI (500 IU/ml solution of activated human thrombin, part of a commercial package sold as a fibrin sealant kit /Tissucol Duo 1.0 mL, (Baxter, Deerfield, IL, USA). The mean PSA diameter was 2.1x1.4 cm. Sixty-four patients with very small PSA cavities (less then 1.5x1.5 cm) suitable for local compressive therapy, or with very big cavities (5x5 cm) scheduled for surgery, were excluded from the thrombin application. The factors associated with 30-days recurrence of PSA were retrospectively analysed.
Cardiac catheterisation and arterial puncture site closure
In the study period, 13,890 heart catheterisations (9,943 diagnostic and 3,947 interventional) were performed via femoral arterial approach. Arterial sheaths size ranged from 5 Fr to 10 Fr. Anti-coagulation during the procedure was accomplished by intravenous un-fractionated heparin. None of the patients received GP IIb/IIIa receptor inhibitors or bivaluridin. Arterial closure was achieved by manual compression alone or in conjunction with a pneumatic compression device (FemoStop™, Radi Medical Systems AB, Uppsala, Sweden). Arterial closure devices were not used in this time period. Patients were ambulated 12 to 24 hours after the catheterisation procedure. A groin check was routinely made after the procedure, before ambulation, and prior to discharge. In the case of a local puncture site complication which was suspicious upon physical examination, a colour-duplex ultrasound (CDUS) control was scheduled.
Colour-duplex ultrasound (CDUS)
The CDUS image of femoral artery PSA was characterised by arterial flow through a narrow neck arising from the puncture place in femoral artery (Figure 1).
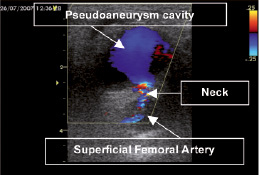
Figure 1. CDUS image of pseudoaneurysm. Arterial flow through a narrow neck arising from the puncture place in femoral artery.
Typical “to-and-fro” Doppler waveform in the PSA neck was an essential criterion for proper diagnosis (Figure 2).
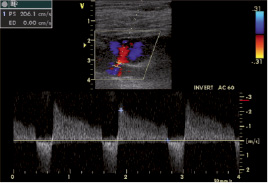
Figure 2. Pulsed-wave Doppler in the neck of pseudoaneurysm. Typical “to-and-fro” Doppler waveform in the PSA neck is essential criterion for proper diagnosis.
Under 2-D ultrasound control, the 20-gauge needle was positioned in the middle of the PSA cavity. Exact position of the needle tip was properly identified before thrombin application. Injection of thrombin was characterised by a sudden thrill, considered a sign of fast blood thrombilisation in the cavity. Complete thrombilisation of the cavity was confirmed by the absence of the flow in the PSA after the thrombin injection. Distal arterial Doppler flow in the superficial femoral artery (SFA) was recorded before and after thrombin application to analyse eventual distal embolisation or arterial thrombosis after cavity occlusion.
Data and statistical analyses
The following clinical factors were evaluated in relation to the occurrence of PSA: obesity, defined as BMI>30, arterial hypertension, presence of coronary artery disease, use of thienopyridines, low-molecular weight heparin (LMWH) anti-coagulation therapy and combination of LMWH+ASA or thienopyridines.
Data evaluation was performed using a statistical software package SPSS 13.0 (SPSS Inc., Chicago, IL, USA). Discrete variables are presented as counts and percentages. Continuous variables are presented as mean values±SD. In all cases, the Kolmogorov-Smirnov test was applied to test for a normal distribution. The frequencies of categorical variables were compared using Fisher´s exact test. Mean values for continuous parameters were compared using the Student t test and Mann-Whitney test, used as appropriate. Multivariate logistic regression analysis was used to study multiple risk factors for PSA recurrence after occlusion by thrombin. For all analyses, a p value <0.05 was considered statistically significant.
Results
The baseline patients characteristics are given in Table 1.
The incidence of femoral PSA occurrence during the studied period was 1.5% (204/13 890). As shown in Table 2, immediate success rate of UGTI was 85% (119/140) – 119 patients were successfully treated by one injection of activated thrombin (average amount 0.4(0.2 ml).

In 19 patients (13.6%), immediate short local compression (two minutes) following the injection was needed for complete occlusion (98.6% overall success rate). In one case, progression of PSA required conversion to surgical repair (0.7%). In one patient with pre-existing significant stenosis of superficial femoral artery, the acute limb ischaemia developed after thrombin application and was successfully treated by pharmaco-mechanical thrombectomy (0.7%). During the 30-days follow-up recurrence of PSA occurred in 10 patients (7%), exclusively the second and the third day after the first injection. All of them were successfully treated by the second thrombin application.
At univariate analysis, obesity (BMI>30, OR=1.39, 95% CI 1.09-1.78, p<0.0001), anti-coagulation therapy by LMWH (OR=1.21, 95% CI 1.07-1.36, p<0.0001), and combination of anti-aggregation and anti-coagulation therapy (ASA or thienopyridines+LMWH), (OR=2.11, 95% CI 1.23-3.62, p<0.0001) were associated with increased risk of PSA recurrence (Table 3, Figure 3).
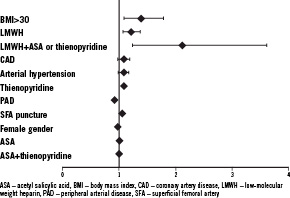
Figure 3. The risk factors of PSA recurrence (OR: odd ratio).
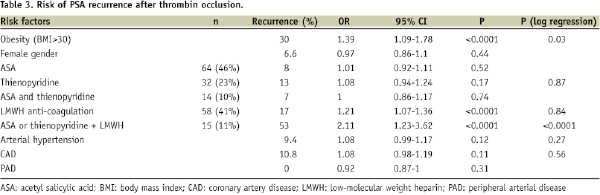
Sheath size did not correlate with PSA recurrence. For multivariate analysis, parameters with p<0.2 based on univariate analysis were included into the final model. At multivariate analysis, the combination therapy of LMWH and ASA or thienopyridines and obesity (BMI>30) emerged as independent risk factors for PSA recurrence after thrombin occlusion (Table 3).
Discussion
The results of the present study can be summarised as follows: 1) the incidence of femoral artery PSA after left heart catheterisation was 1.5%; 2) immediate success rate of PSA occlusion by UGTI is high with very low complication rate of thrombin application; 3) the recurrence of PSA after UGTI was as low as 7%, and was associated with obesity and extensive combination of anti-aggregation and anti-coagulation therapy.
Definition and management of PSA
An arterial PSA is a cavity or pulsatile haematoma caused by disruption of the arterial wall at the site of puncture with extra-luminal blood flow into the chamber composed by adjacent tissue. Typical risk factors relate to obesity and technical aspects of the catheterisation such as inadequate manual compression after removal of the arterial sheath, sheath size, or the larger number of cases performed per day in a particular room10. The risk factors, namely diabetes mellitus or hypertension appear to be associated with PSA10. In addition, advanced age and a platelet count of less than 200x109/L emerged as risk factors for PSA2,4,11.
The localised pain in the groin, its expansion and pulsatility, bruit, drop in haemoglobin level, and pronounced limp, are main clinical characteristics of femoral artery PSA. The PSA could be complicated by thromboembolism, extrinsic compression of surrounding structures, necrosis of the skin and subcutaneous tissue, and rarely by arterial wall rupture. Significant blood loss can result in haemorrhagic shock.
The large PSA have been traditionally repaired by surgery with the risk of complications such as wound infection, lymphocele or chronic lymphoedema. The smaller cavities were typically managed by observation and prolonged local compression. However, difficulties in prediction of spontaneous PSA thrombosis, patient’s discomfort, the necessity of repeated ultrasound controls, and subsequent prolongation of hospitalisation make this approach more expensive and less practical. The ultrasound-guided compression specifically in the origin of the PSA neck is more effective approach, however it is time consuming, and often associated with the pain despite the local anaesthesia. In addition, the failure rate of ultrasound-guided compression was reported to be 5-15%12,13, with high recurrence in patients with concomitant anti-coagulation therapy13,14.
Thrombin application
Thrombin is a potent enzyme transforming fibrinogen to fibrin in the cascade of blood coagulation. The high effectiveness, but also the safety of thrombin application in patients with iatrogenic PSA formation are the basis for the widespread thrombin use for PSA occlusion in the last years1,3,4,8,9.
Acute arterial occlusion is the rare complication of UGTI mostly attributed to the wide and short neck of PSA cavity15,16. In our case of a single patient with acute SFA occlusion, the thrombotic complication was related to the pre-existing stenosis in the mid segment of the superficial femoral artery. Thrombosis was successfully treated by pharmaco-mechanical thrombectomy using the AngioJet thrombectomy system (Possis Medical, Inc., Minneapolis, MN, USA). Thus, significant stenosis of the femoral artery distally to the PSA should be considered as a contraindication of thrombin application and careful duplex evaluation distal to the pseudoaneurysm is warranted prior to thrombin application.
In all patients of our study, the human thrombin was applied. Bovine thrombin injection was described to be associated with higher number of injections required for PSA closure17 as well as with potential anaphylaxis after repeated exposures to bovine thrombin18. Additionally, hypotension and bradycardia are potential reactions to exposure to bovine thrombin19.
The PSA recurrence
Our study presents the large cohort of consecutive patients presenting with PSA and treated with thrombin injections and confirm the low rates of PSA recurrence. This extends the findings reported in earlier study of Olsen et al who reported the 6% recurrence rate of PSA after thrombin occlusion1. On the contrary, Sackett et al did not report the recurrence of PSA during the 30-days follow-up of 27 patients and anti-coagulation therapy did not affect the results of thrombin application. Eleven patients from this cohort were systemically anti-coagulated with heparin or warfarin at the time of UGTI.20 Likewise, La Perna et al and Schneider et al concluded that anti-coagulant use did not hinder successful thrombosis after UGTI3,21. Among 70 patients treated by Le Perna et al, 21 patients (30%) were receiving antithrombotic therapy with anti-coagulation at the time of the procedure. The majority of the others were also on dual antiplatelet therapy. The UGTI was successful in 66 of the 70 patients (94%), however three patients showed early PSA recurrence within 24 hours. Note, the average dose of reconstituted bovine thrombin was 1.15 ml (1150 U) in the study of Le Perna et al3 and 0.6 ml of 1000 IU/ml in the cohort of Sackett et al20 as compared to our study (0.4 ml from 500 IU/ 1ml of human thrombin).
Our data confirmed no significant risk of PSA recurrence after UGTI associated with anti-coagulation therapy. However, the combination of LMWH anti-coagulation with anti-aggregation medication (ASA or thienopyridines) emerged as an independent risk factor of PSA recurrence. None of our patients received dual anti-aggregation therapy in combination with anti-coagulation. It should be noted, that our results could have been affected by the dosage and concentration of applied human thrombin (0.4±0.2ml of 500 IU/ml solution).
Accordingly, the optimal patient suitable for UGTI of femoral PSA is one with diameter of cavity approximately 1 cm or larger, without acute symptoms of rapid PSA progression, and without severe stenosis of femoral artery distally to the PSA. Cavities smaller then 1 cm tend towards spontaneous thrombilisation, patients with rapid PSA progression are candidates for urgent surgery. Temporary reduction of LMWH and ASA/thienopyridine combination therapy prior to UGTI is reasonable.
Conclusion
Ultrasound-guided thrombin injection is a safe and effective treatment option of iatrogenic femoral artery pseudoaneurysm. It should be considered as a method of choice in suitable patients. Low rates of recurrence are associated with obesity and extensive use of combined anti-aggregation and anti-coagulation therapy.

