Abstract
Background: Vascular complications still represent an important issue after transcatheter aortic valve implantation (TAVI).
Aims: The aim of this study was to evaluate the effectiveness of upfront use of an adjunctive Angio-Seal (AS) plug-based system on top of suture-based devices (SBDs) for endovascular haemostasis after transfemoral (TF) TAVI.
Methods: From January 2019 to April 2020, 332 consecutive patients with preprocedural computed tomography angiography (CTA) assessment underwent fully percutaneous TF-TAVI. The primary outcomes were 30-day major vascular complications and major or life-threatening (LT) bleeding due to endovascular closure system failure. A total of 246 TF-TAVI patients (123 pairs), undergoing either isolated SBD or SBD+AS, were matched using the propensity-score method.
Results: At 30 days, patients receiving SBD+AS had lower rates of major/LT bleeding (1.6% vs 8.9%, odds ratio [OR] 0.17, 95% confidence interval [CI]: 0.04-0.78; p<0.01) and major vascular complications (1.6% vs 8.9%, OR 0.17, 95% CI: 0.04-0.78; p<0.01). In addition, the use of SBD+AS was associated with a significant cost saving related to the vascular event (mean difference –315.3 € per patient, 95% CI: –566.4 € to –64.1 €; p=0.01), and a higher probability of next-day discharge (NDD) after TAVI (30.9% vs 16.3%, OR 2.30, 95% CI: 1.25-4.25; p<0.01). No difference in all-cause 30-day mortality was observed (3.3% vs 1.6% for SBD and SBD+AS groups, respectively, OR 0.49, 95% CI: 0.09-2.74; p=0.41).
Conclusions: An upfront combined strategy with an additional AS plug-based device on top of SBDs was shown to reduce major vascular complications and major/LT bleeding due to closure system failure after TF-TAVI. This approach was associated with a cost saving and with a higher probability of NDD compared to the use of isolated SBD.
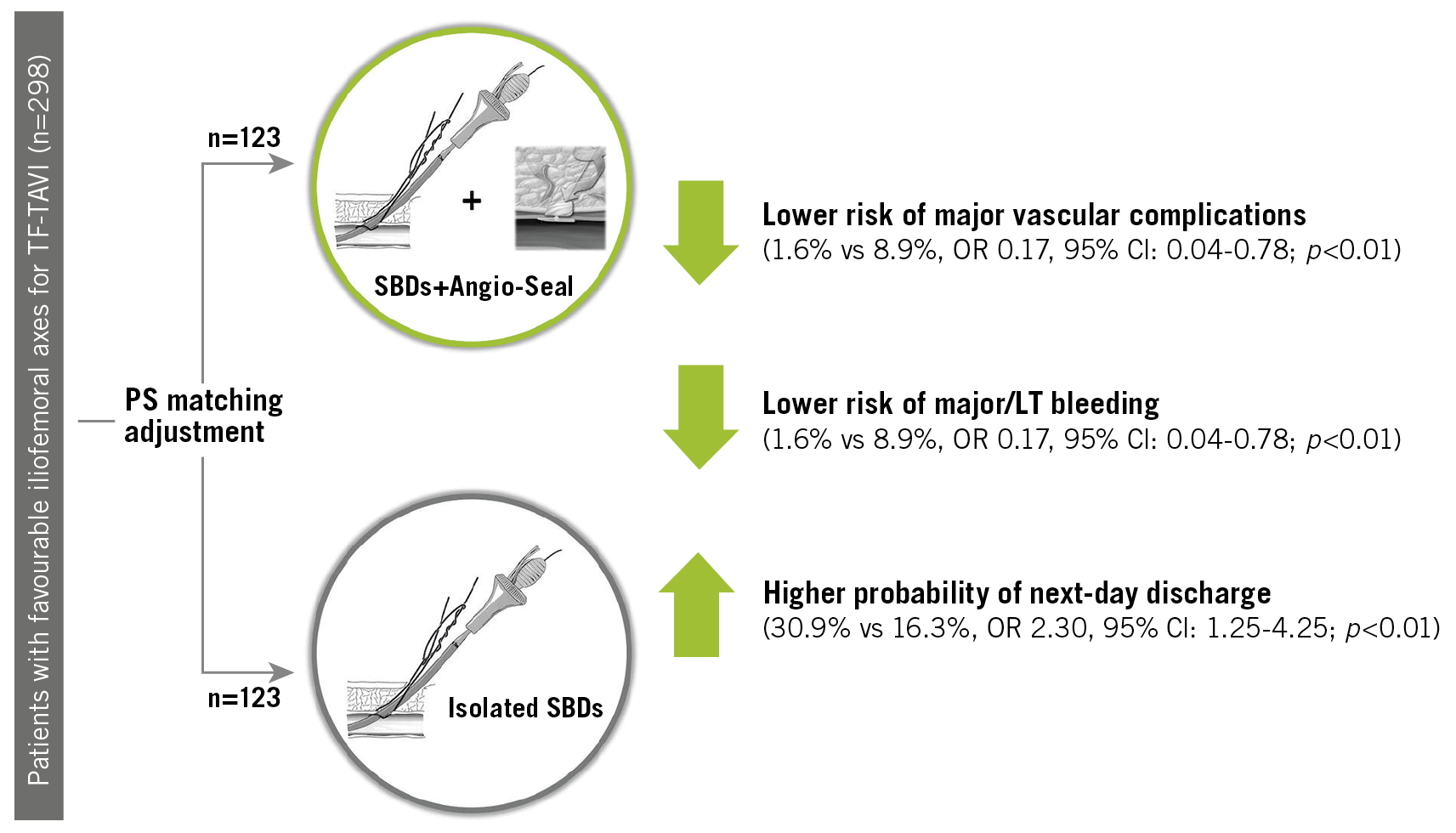
Visual summary. Effectiveness of the upfront combined strategy for endovascular haemostasis in transfemoral transcatheter aortic valve implantation using Angio-Seal on top of a suture-based device (SBD) versus the isolated use of SBD. LT: life-threatening; TF-TAVI: transfemoral transcatheter aortic valve implantation
Introduction
Vascular complications following transfemoral transcatheter aortic valve implantation (TF-TAVI) have been reducing over the years thanks to the growing expertise of operators and refinements in transcatheter systems1. Currently, suture-based devices (SBDs) are the most frequently used endovascular systems to obtain femoral access haemostasis after large-bore arteriotomy2. Nevertheless, rates of major vascular complications and bleeding still represent an important issue that impacts on patients’ early recovery and mobilisation, as well as on midterm prognosis3,4,5,6. In this setting, the aim of our study was to assess the effectiveness of upfront use of an adjunctive Angio-Seal™ (AS) plug-based system (Terumo Corp., Tokyo, Japan) on top of SBDs for endovascular haemostasis after TF-TAVI in terms of reduction of access-related complications due to endovascular closure system failure, and therefore of resource utilisation and cost reduction.
Methods
STUDY POPULATION
This is a single-centre, retrospective analysis obtained from a prospective local TAVI registry7. Consecutive patients undergoing fully percutaneous TF-TAVI with preprocedural computed tomography angiography (CTA) assessment at our institution from January 2019 to April 2020 were included. Femoral arteriotomy was performed percutaneously with angiographic guidance in all patients. The arteriotomy site had been identified previously by preprocedural CTA scans in order to select the most favourable iliofemoral axis in terms of vessel tortuosity (<90°), diameter and calcification. In particular, patients with calcium in the femoral artery anterior wall or circumferential calcification greater than 180°, as well as a sheath-to-femoral artery ratio (SFAR) higher than 1.0, were excluded. Femoral arteriotomy was performed at a site 1-2 cm above the femoral bifurcation, that is no higher than half the femoral head, as per standard practice. Femoral access arteriotomy, percutaneous closure technique and vascular complication management were performed by two operators with more than ten years of expertise in TAVI. The study participant flow is shown in Figure 1.
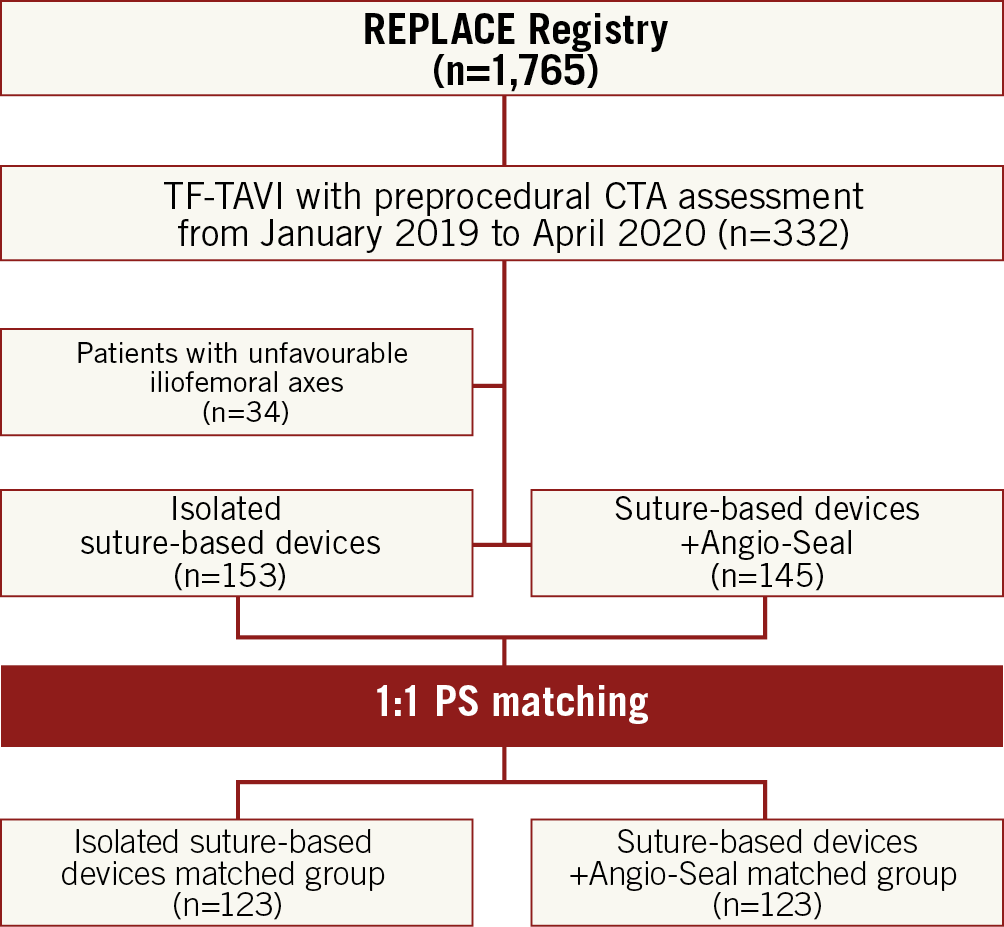
Figure 1. Flow chart of participants.
All study management activities including data management and statistical analyses were performed at the Policlinico-San Marco Hospital, Catania, Italy. All subjects provided written informed consent for the procedure. The study was conducted according to the principles of the Declaration of Helsinki and Good Clinical Practice. The study did not undergo ethics committee approval, considering the retrospective nature of the analysis. The authors wrote all drafts of the paper and vouch for the integrity and the completeness of the data and analyses.
CLOSURE TECHNIQUE
Either single Prostar® XL or dual Perclose ProGlide® SBDs (both Abbott Vascular, Santa Clara, CA, USA) were used for obtaining common femoral artery (CFA) haemostasis. SBDs for endovascular closure were pre-implanted before insertion of transcatheter aortic valve (TAV) sheaths, as per standard practice. At the end of the TAVI procedure, the transcatheter systems were pulled out, leaving the stiff guidewire inside, and one of the two pre-implanted knots was gently pushed towards the CFA, without tightening, in order to reduce actual bleeding. At this point, the AS was advanced through the in situ guidewire into the artery. The addition of the AS plug-based device on top of SBDs was implemented regardless of the residual bleeding. Then, SBD knots were tightened over the AS device and therefore the collagen-based plug was released. Finally, SBD knots were re-tightened after AS system removal. The step-by-step implantation technique is illustrated in Moving image 1 and Figure 2. In order to assess vascular complications after TF-TAVI properly, a final digital subtraction angiography of the femoral artery was performed in left anterior oblique (LAO) and right anterior oblique (RAO) projections, as per standard practice in our institution.
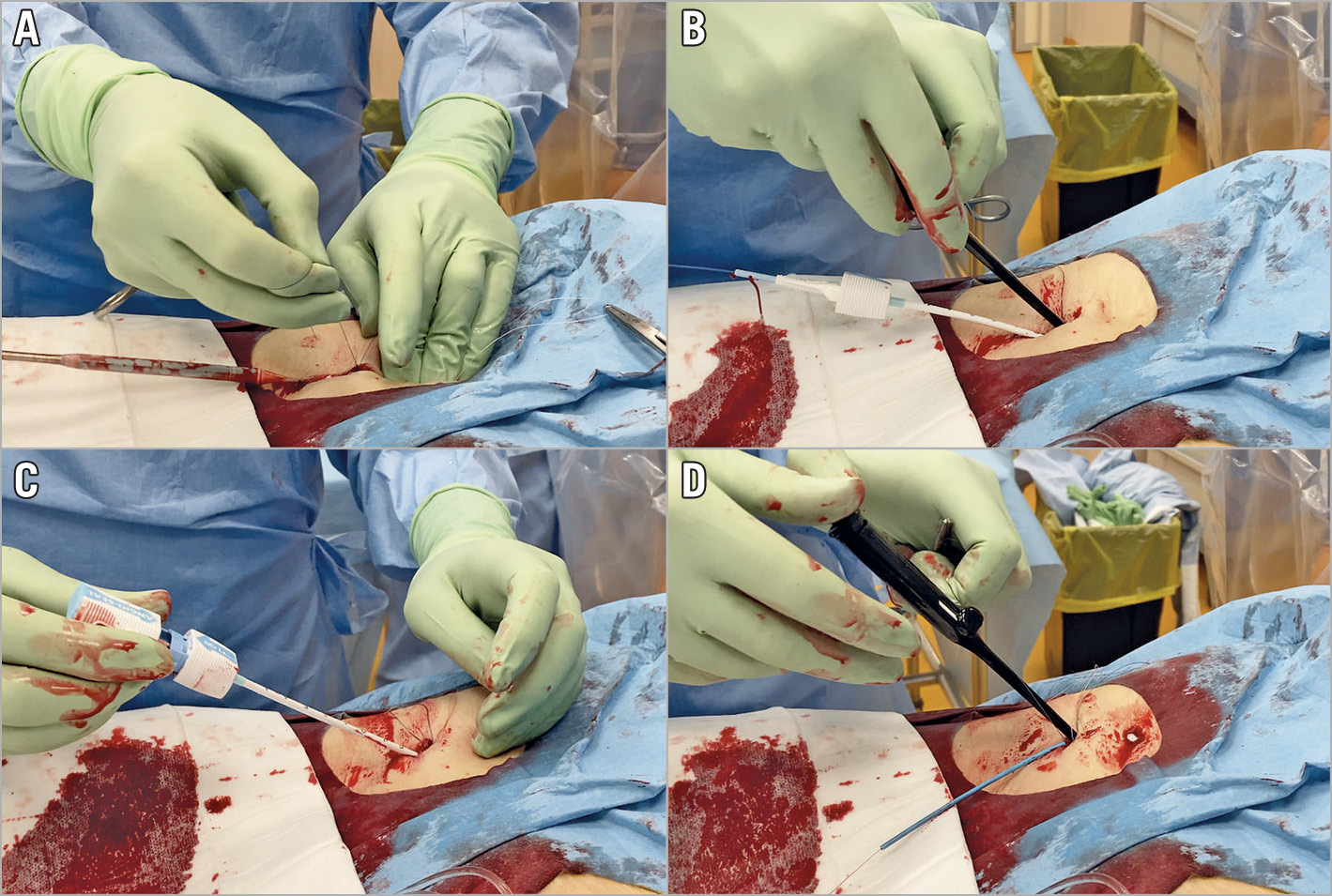
Figure 2. Step-by-step technique of upfront addition of an AS plug-based system on top of an SBD for endovascular haemostasis in TF-TAVI. First, one of the SBD’s knots is gently pushed towards the CFA without tightening, at delivery system withdrawal (A). Then, the AS device is advanced inside the artery on the in situ, stiff guidewire and both of the SBD’s knots are tightened on it (B). Afterwards, the AS is implanted (C) and the SBD’s knots are finally re-tightened (D).
STUDY ENDPOINTS
The primary outcomes were major vascular complications and major or life-threatening (LT) bleeding due to endovascular closure system failure at 30 days.
A specific cost analysis was performed taking into account only additional costs related to vascular complications.
First, we compared the 30-day outcomes of patients undergoing TF-TAVI and receiving only SBD or SBD+AS for endovascular closure considering the entire population. Then, we accounted for any confounding variables between the two groups through a propensity score (PS) matching and re-assessed the outcomes of the SBD and SBD+AS matched groups. All outcomes were reported according to Valve Academic Research Consortium-2 definitions8.
STATISTICAL ANALYSIS
Continuous variables were reported as median and interquartile range (IQR), whereas dichotomous parameters were reported as frequencies and percentages. To account for the non-normal distribution of data, baseline characteristics and clinical outcomes before and after matching were analysed using Fisher’s exact test and the Mann-Whitney U test between the two groups, as appropriate. Odds ratio (OR) estimates were reported with their 95% confidence interval (CI). To adjust for potential bias in treatment assignment, two groups of patients with similar preprocedural characteristics were selected using PS matching by logistic regression with the nearest neighbour method. A non-parsimonious approach was used in order to include all potential factors that could influence treatment9,10. Variables included were age, sex, hypertension, diabetes, Society of Thoracic Surgeons (STS) mortality score, body mass index (BMI), renal failure (defined as estimated glomerular filtration rate <30 ml/min/m2 according to the Cockcroft-Gault formula).
The matching algorithm used in this analysis was implemented in the PS Matching package version 3.0.4 (SPSS; IBM Corp., Armonk, NY, USA), details of which have been reported elsewhere.
A two-step analysis was used to assess predicting factors. First, a single logistic regression was performed. Afterwards, a stepwise forward selection method was used for multivariable logistic regression analysis with a threshold of p<0.10 and p<0.05 for entering into and remaining in the model, respectively.
A sensitivity analysis was performed excluding patients treated with dual Perclose ProGlide SBDs.
All statistical tests were performed two-tailed, and a significance level of p<0.05 was considered to indicate statistical significance. The statistical software package IBM SPSS Statistics, Version 25.0 (IBM Corp.) was used for all statistical analyses.
Results
BASELINE AND PROCEDURAL CHARACTERISTICS
From January 2019 to April 2020, a total of 298 consecutive patients with favourable iliofemoral axes at preprocedural CTA assessment underwent TF-TAVI in our institution. Patients had a mean age and STS mortality score of 80.5±6.8 years and 3.7±2.2%, respectively, and received the Prostar XL SBD for endovascular closure in most cases (92.3%). One hundred and fifty-four patients (51.7%) were treated with an isolated SBD, whereas 145 patients (48.3%) were treated with an SBD+AS combined strategy.
At baseline, pre-existing atrial fibrillation (AF) (24.8% vs 13.1%, p<0.01) was more frequent in patients treated with an isolated SBD.
After PS matching, 123 pairs of patients receiving an SBD or SBD+AS for endovascular haemostasis were included in the main analysis. No differences in baseline and procedural characteristics were encountered between the two matched groups. Baseline and procedural characteristics, before and after PS matching, are shown in Table 1 and Table 2, respectively.
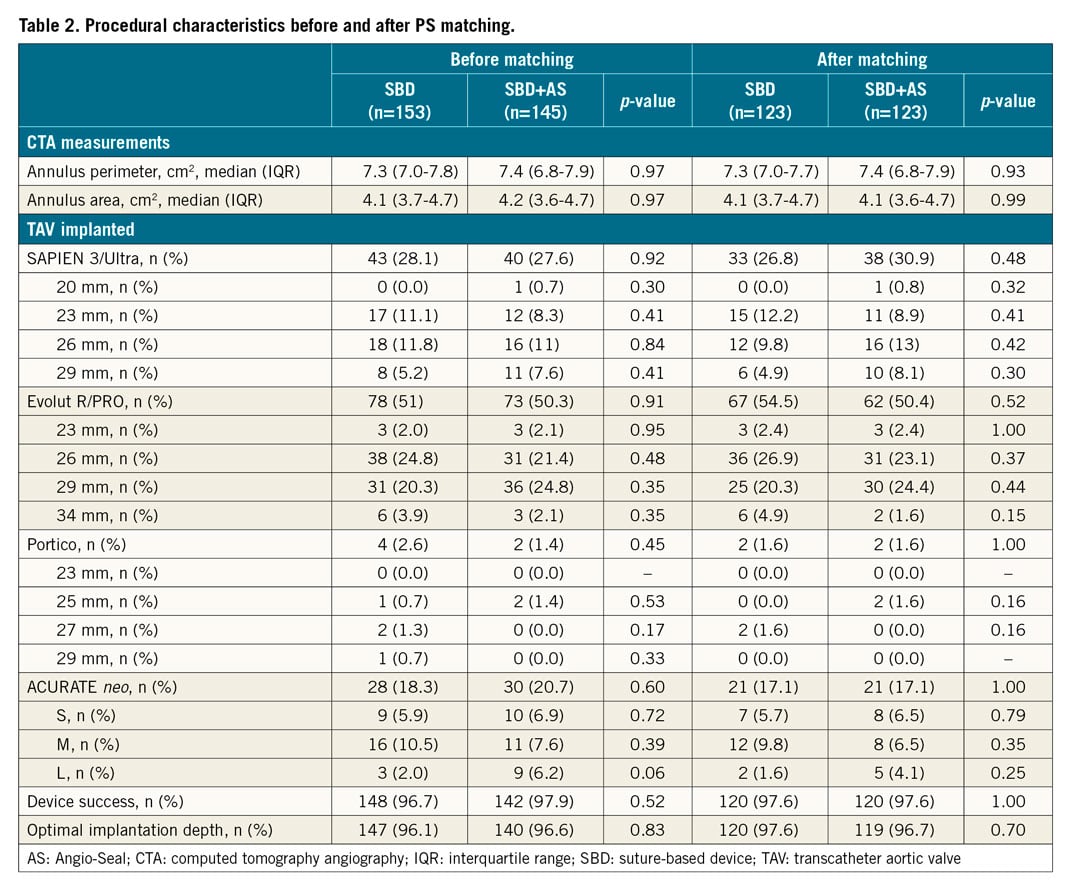
THIRTY-DAY OUTCOMES
Thirty-day outcomes, before and after PS matching, are reported in Supplementary Table 1 and Table 3, respectively.
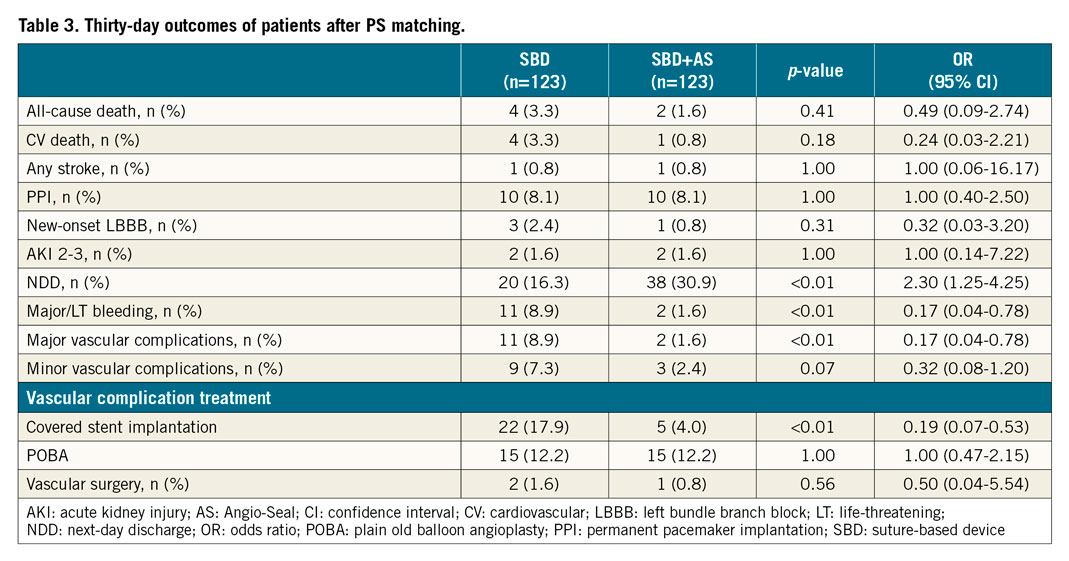
Vascular problems after endovascular closure of primary TAVI access were related to residual bleeding with or without concomitant femoral artery dissection in all cases (Supplementary Table 2). No residual arterial stenosis was encountered. After PS matching, reference vessel diameter (RVD) (7.5 [5.8-9.2] vs 7.3 [5.7-9.1] mm for SBD and SBD+AS groups, respectively; p=0.76) and SFAR values (0.68 [0.50-0.85] vs 0.69 [0.52-0.86] for SBD and SBD+AS groups, respectively; p=0.92) did not differ between the groups. Patients receiving SBD+AS had a significantly lower risk of major/LT bleeding (8.9% vs 1.6%, OR 0.17, 95% CI: 0.04-0.78; p=0.01), and major vascular complications (8.9% vs 1.6%, OR 0.17, 95% CI: 0.04-0.78; p=0.01) at 30 days (Figure 3). Furthermore, the SBD group showed a higher rate of percutaneous transluminal angioplasty (PTA) of the CFA with covered stent implantation (4.1% vs 17.9%, p<0.01). No differences in terms of all-cause mortality (3.3% vs 1.6%, for SBD and SBD+AS matched groups, respectively, OR 0.49, 95% CI: 0.09-2.74; p=0.41) and cardiovascular mortality (3.3% vs 0.8%, for SBD and SBD+AS matched groups, respectively, OR 0.24, 95% CI: 0.03-2.21; p=0.18), any stroke (0.8% vs 0.8%, for SBD and SBD+AS matched groups, respectively, OR 1.00, 95% CI: 0.06-16.17; p=1.00), and permanent pacemaker implantation (PPI) (8.1% vs 8.1%, OR 1.00, 95% CI: 0.40-2.50; p=1.00) were reported between matched groups at 30 days.
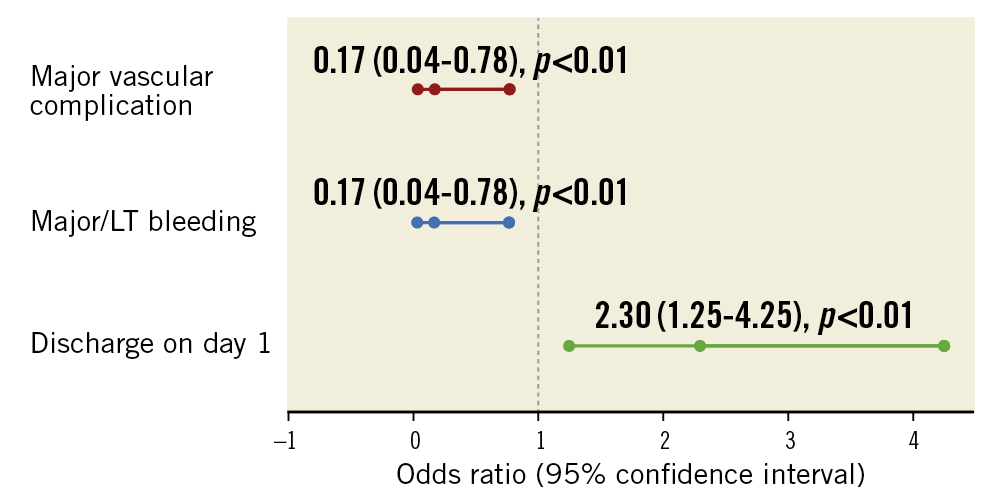
Figure 3. Key outcomes associated with the use of a combined SBD+AS strategy after PS matching.
POST-PROCEDURAL LENGTH OF STAY AND COST ANALYSIS
After TAVI, patients were transferred directly to a general ward in most cases (97.7%). Median length of stay (LoS) after TAVI was 2 days (interquartile range [IQR] 2-4 days) and median additional cost due to a vascular event was 114,00 € per patient (IQR 0-114,00 €).
After PS matching, patients receiving SBD+AS had a higher probability of being discharged the day after the procedure (30.9% vs 16.3%, OR 2.30, 95% CI: 1.25-4.25; p<0.01) (Table 3, Figure 3), and showed a significant cost reduction related to vascular events (mean difference –315.30 € per patient, 95% CI: –566.40 to –64.10 €; p=0.01).
Finally, in an exploratory analysis aimed at identifying predictors of next-day discharge (NDD), the use of a combined strategy with SBD+AS for endovascular haemostasis (OR 2.23, 95% CI: 1.28-3.89; p<0.01), as well as baseline blood haemoglobin value (OR 1.18, 95% CI: 1.01-1.38; p=0.03) and post-procedural PPI (OR 0.09, 95% CI: 0.01-0.68; p=0.03), were confirmed to be independently associated with this endpoint (Supplementary Table 3).
Discussion
The optimisation of preprocedural planning by CTA assessment and the improvements of new-generation TAV delivery systems, as well as the consolidated experience of operators with endovascular SBDs, have been contributing to lowering the rates of vascular complications and bleeding after TAVI over recent years1.
Nevertheless, a non-negligible percentage of patients still experience vascular complications due to SBD failure after transfemoral TAVI, which could impact on midterm prognosis.
The aim of the present analysis was to evaluate the effectiveness of the upfront use of an additional AS plug-based device on top of SBDs in patients undergoing TF-TAVI, comparing this novel strategy to the standard use of an isolated SBD for endovascular haemostasis.
The main findings of our study are: 1) vascular complications and bleeding due to SBD failure still represent an important issue, even avoiding unfavourable vascular access sites by proper preprocedural CTA assessment; 2) a strategy of upfront addition of an AS plug-based device on top of SBDs was shown to lower vascular complications and bleeding significantly after transfemoral TAVI; 3) this strategy was demonstrated to reduce hospital resource utilisation by reducing the LoS and lowering procedure-related costs.
Although the extensive use of preprocedural CTA assessment has allowed identification of several anatomical and procedural predictors of vascular complications after TF-TAVI over the past decade11, SBDs for endovascular closure have shown a non-negligible percentage of failure, which results in various grades of vascular complications. In this setting, a dual ProGlide strategy seems to have better results in terms of major vascular complications compared to single Prostar XL use, even if the real benefit of a given SBD over the other is still debated2,12,13,14.
In our analysis, we considered only patients undergoing transfemoral TAVI, who did not have unfavourable iliofemoral characteristics at preprocedural CTA assessment, and who were treated with either isolated SBDs or by combining an SBD with the upfront addition of an AS plug-based device for obtaining endovascular closure. Considering the entire population, 30-day rates of all-cause mortality, major vascular complications related to closing system failure and major or LT bleeding after TAVI were 2.7%, 5.4% and 5.4%, respectively, which are in line with those reported in large, prosthesis-specific, TF-TAVI registries15,16,17,18 (Figure 4).
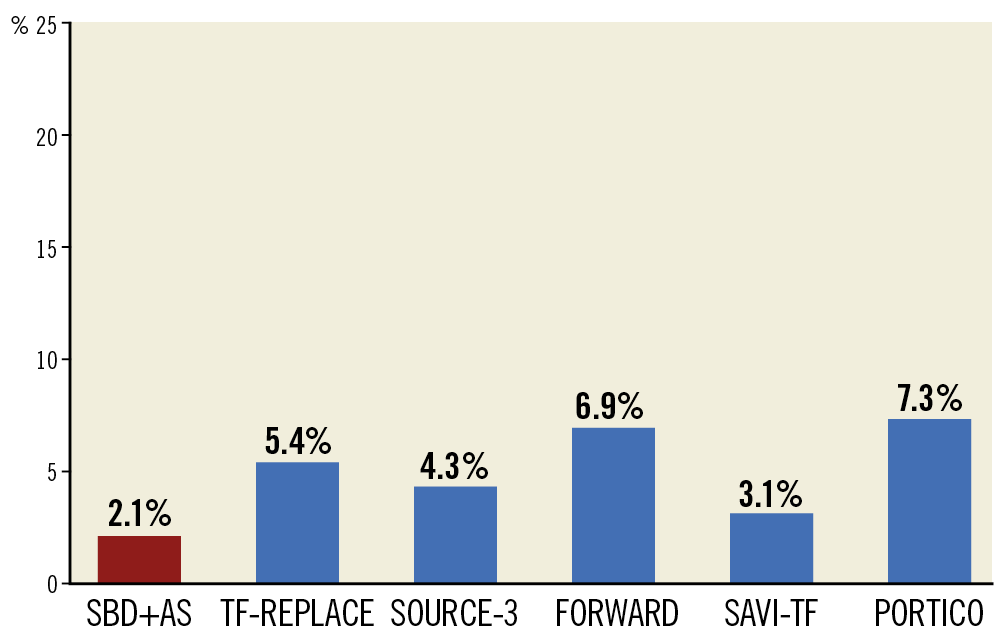
Figure 4. Rates of major vascular complications in large, prosthesis-specific, TF-TAVI registries compared to the overall rate in the TF-REPLACE registry and to the rate of patients treated with a combined SBD+AS strategy (red bar).
The use of an additional AS on top of a dual ProGlide strategy has been investigated previously in a retrospective study19. It reported that this strategy was safe and feasible for obtaining complete haemostasis after TAVI, even if there was no clear benefit in terms of vascular complications and bleeding compared to the use of an isolated dual ProGlide strategy. Nevertheless, this study suffered from important limitations: an AS was added in case of residual bleeding at the discretion of the operators and patients were not selected according to the CTA characteristics of iliofemoral vessels. Also, patient groups were not adjusted to take into account any confounding variables.
In our study, after PS matching, we reported significant differences between the use of an isolated SBD and the upfront addition of an AS plug-based device on top of SBDs in terms of vascular complications and bleedings after TAVI. In particular, an SBD+AS combined strategy showed a significantly lower risk of major vascular complications (8.9% vs 1.6%, OR 0.17, 95% CI: 0.04-0.78; p=0.01) and major/LT bleeding (8.9% vs 1.6%, OR 0.17, 95% CI: 0.04-0.78; p=0.01) at 30 days (Figure 3, Figure 4). As a corollary finding, patients receiving SBD+AS for endovascular closure underwent femoral PTA with covered stent implantation in a lower percentage of cases (4.1% vs 17.9%, p<0.01). However, the difference in rates of vascular events did not affect overall mortality (3.3% vs 1.6% for SBD and SBD+AS groups, respectively; p=0.41).
Vascular complications and bleeding after TAVI significantly affect post-procedural in-hospital care20,21. Although the majority of vascular events can be recognised immediately and solved during the index procedure by endovascular treatment, the presence of a significant haemoglobin drop at post-procedural blood exams may raise concerns about any further unnoticed vascular damage during TAVI. As a consequence, post-procedural LoS and resource utilisation increase after TAVI.
In this setting, our findings showed that the benefit of the combined use of SBD+AS for endovascular closure, in terms of reduction of vascular complications and bleeding, is in turn reflected in a significant vascular complication-related cost saving of approximately 315 € per patient. In addition, patients treated with SBD+AS had a more than twofold probability of having a faster recovery and of being discharged the day after TAVI (30.9% vs 16.3%).
Limitations
The main limitation of our study lies in its single-centre, retrospective design with a relatively small sample size. Furthermore, although the adjustment with PS matching has guaranteed an optimal balancing of baseline variables between study cohorts, a selection bias cannot be excluded due to the non-randomised nature of the study. Finally, we did not assess the distance between the common femoral artery and the patient’s skin, which could differ between the two groups. In addition, we used mainly the Prostar XL device in our TAVI practice, even if the rationale of integrating different mechanisms to improve artery haemostasis did not differ between the Prostar XL and the dual ProGlide.
Conclusions
An upfront combined strategy with an additional AS plug-based device on top of SBDs was shown to reduce major vascular complications and major/LT bleeding after TF-TAVI. This approach was associated with a cost saving and with a higher probability of being discharged the day after the procedure compared to the use of an isolated SBD. Due to the retrospective nature of this analysis, the results need to be confirmed in larger, prospective, randomised studies.
|
Impact on daily practice Although vascular complications following transfemoral trans-catheter aortic valve implantation (TF-TAVI) have been reducing over the years thanks to the growing expertise of operators and refinements of transcatheter systems, the rates of major vascular complications and bleeding still represent an important issue, which impacts on patients’ early recovery and mobilisation, as well as on midterm prognosis. In our study, we demonstrated that a combined strategy for endovascular closure after TF-TAVI with the upfront addition of a collagen-based plug system on top of suture-based devices (SBDs) was shown to lower the risk of major vascular complication and major/life-threatening bleeding, as well as length of stay and additional costs related to vascular events, compared to the use of isolated SBDs. |
Guest Editor
This paper was guest edited by Franz-Josef Neumann, MD; Department of Cardiology and Angiology II, University Heart Center Freiburg - Bad Krozingen, Bad Krozingen, Germany.
Conflict of interest statement
M. Barbanti is a consultant for Edwards Lifesciences and is an advisory board member for Boston Scientific and Medtronic. C. Tamburino has received speaker honoraria from Medtronic, Abbott Vascular, Edwards Lifesciences and Boston Scientific. The other authors have no conflicts of interest to declare. The Guest Editor reports lectures fees paid to his institution from Amgen, Bayer Healthcare, Biotronik, Boehringer Ingelheim, Boston Scientific, Daiichi Sankyo, Edwards Lifesciences, Ferrer, Pfizer, and Novartis, consultancy fees paid to his institution from Boehringer Ingelheim, and grant support from Bayer Healthcare, Boston Scientific, Biotronik, Edwards Lifesciences, GlaxoSmithKline, Medtronic, and Pfizer.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Step-by-step technique of the upfront addition of an AS plug-based system on top of an SBD for endovascular haemostasis in TF-TAVI.

