Abstract
Objectives: The aim of the current Valvular Academic Research Consortium (VARC)-2 initiative was to revisit the selection and definitions of transcatheter aortic valve implantation (TAVI)- clinical endpoints to make them more suitable to the present and future needs of clinical trials. In addition, this document is intended to expand understanding of patient risk stratification and case selection.
Background: A recent study confirmed that VARC definitions have already been incorporated into clinical and research practice and represent a new standard for consistency in reporting clinical outcomes of patients with symptomatic severe aortic stenosis (AS) undergoing TAVI. However, as the clinical experience with this technology has matured and expanded, certain definitions have become unsuitable or ambiguous.
Methods and results: Two in-person meetings (held in September 2011 in Washington, DC, USA, and in February 2012 in Rotterdam, The Netherlands) involving VARC study group members, independent experts (including surgeons, interventional and non-interventional cardiologists, imaging specialists, neurologists, geriatric specialists, and clinical trialists), the United States Food and Drug Administration (FDA), and industry representatives, provided much of the substantive discussion from which this VARC-2 consensus manuscript was derived. This document also provides an overview of risk assessment and patient stratification that needed to be considered for accurate patient inclusion in studies. Working groups were assigned to define the following clinical endpoints: mortality, stroke, myocardial infarction, bleeding, acute kidney injury, vascular complications, conduction disturbances & arrhythmias, and a miscellaneous category including relevant complications not previously categorized. Furthermore, comprehensive echocardiographic recommendations are provided for evaluation of prosthetic valve (dys)function. Definitions for quality of life assessments are also reported. These endpoints formed the basis for several recommended composite endpoints.
Conclusions: This VARC-2 document has provided further standardization of endpoint definitions for studies evaluating the use of TAVI, which will lead to improved comparability and interpretability of study results, supplying an increasingly growing body of evidence with respect to transcatheter aortic valve implantation and/or surgical aortic valve replacement. This initiative and document can furthermore be used as a model during current endeavors of applying definitions to other transcatheter valve therapies (for example, mitral valve repair).
Introduction
The first Valve Academic Research Consortium (VARC) consensus manuscript was published in January 2011 with the goal of achieving consensus for (1) selecting appropriate clinical endpoints reflecting device, procedure and patient-related effectiveness and safety, and (2) standardizing definitions for single and composite clinical endpoints, for transcatheter aortic valve implantation (TAVI) clinical trials1,2. A recent pooled analysis, which included 3,519 patients from 16 unique studies, confirms that VARC definitions have already been incorporated into clinical and research practice and represent a new standard for consistency in reporting clinical outcomes of patients with symptomatic severe aortic stenosis (AS) undergoing TAVI3. However, as the clinical experience with this technology has matured and expanded, certain definitions have become unsuitable or ambiguous3-7. The aim of the current VARC was therefore to revisit the selection and definitions of TAVI-related clinical endpoints to make them more suitable to the present and future needs of clinical trials. In addition, this document is intended to expand understanding of patient risk stratification and case selection.
Similar to the VARC-1 process, two in-person meetings (held in September 2011 in Washington, DC, USA, and in February 2012 in Rotterdam, The Netherlands) involving VARC study group members, independent experts (including surgeons, interventional and non-interventional cardiologists, imaging specialists, neurologists, geriatric specialists, and clinical trialists), the United States Food and Drug Administration (FDA), and industry representatives, provided much of the substantive discussion from which this VARC-2 consensus manuscript was derived (See Appendix).
Risk scores and comorbidities
Risk stratification of patients is crucial to identify appropriate candidates for specific cardiac procedures. The EuroSCORE and Society of Thoracic Surgeons (STS) score are the most widely used risk scores to predict operative mortality in cardiac surgery. These models were developed and validated in a standard surgical risk population. The predictive power of both models is therefore suboptimal in high-risk patients with valvular disease, although the STS score has shown to outperform the Logistic EuroSCORE8. These models are even more limited in application to patients who are considered prohibitive risk for cardiac surgery, a cohort of great relevance for TAVI. Current models could be improved by the addition of specific clinical and anatomical variables that affect mortality9. As an example, the presence of a porcelain aorta and frailty are important factors not included in either risk model but are routinely considered during patient evaluation (Figure 1 and Table 1).
Perhaps the most important patient characteristic not included in current risk models is “frailty”10. Frailty is frequently assessed subjectively based upon an informal “eyeball test”. However, physical performance assessments such as gait speed and grip strength are more objective performance measures that may capture an individual’s overall functional status11. These continuous measures are reproducible and can be re-assessed at various time points. In addition, they require no language translation. Assessments of cognition, weight (loss), activity level, and independence in activities of daily living provide additional information on the overall health state of the individual11. These limitations are more often found in patients with high comorbidity burden and may coexist with certain laboratory findings (e.g., low serum albumin, elevated inflammatory markers, anaemia) that further reflect the health state and physiologic reserve of the frail patient.
Baseline evaluation of the presence of cognitive dysfunction (mild cognitive impairment or dementia) has also emerged as an essential part of the initial risk stratification, especially in older populations, where risk, benefit, and cost-effectiveness of invasive procedures must be weighed judiciously. Pre-procedural cognitive assessment may also help avoid attributing post-procedural mental status changes to stroke categories. Among the several clinically established rating scales (e.g. mini-mental state examination (MMSE), modified Telephone Interview of Cognitive Status (TICS-M), Clinical Dementia Rating Scale)12, there is no particular standard for TAVI. Nevertheless, some systematic cognitive assessment by neuropsychological experts should be a part of the initial heart team evaluation.
Table 1 provides an overview of these and other risk factors (Figure 1, Figure 2 and Figure 3) and VARC-2 recommendations on how each should be assessed. In clinical trials, it will be important to capture reasons for extreme operative risk and to standardize the evaluation criteria and process. This will help to determine which subsets of patients are likely to benefit from TAVI treatment.
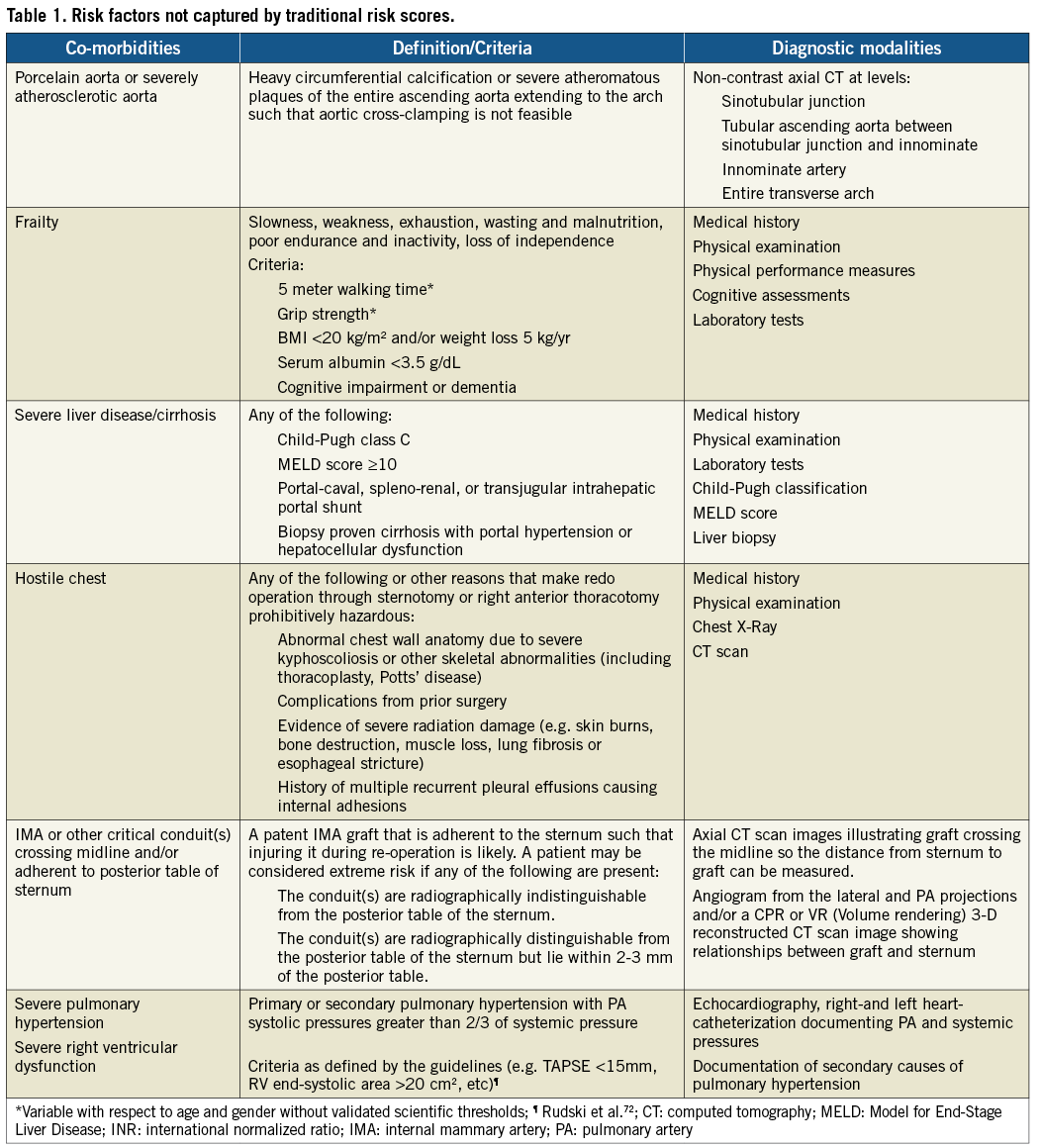
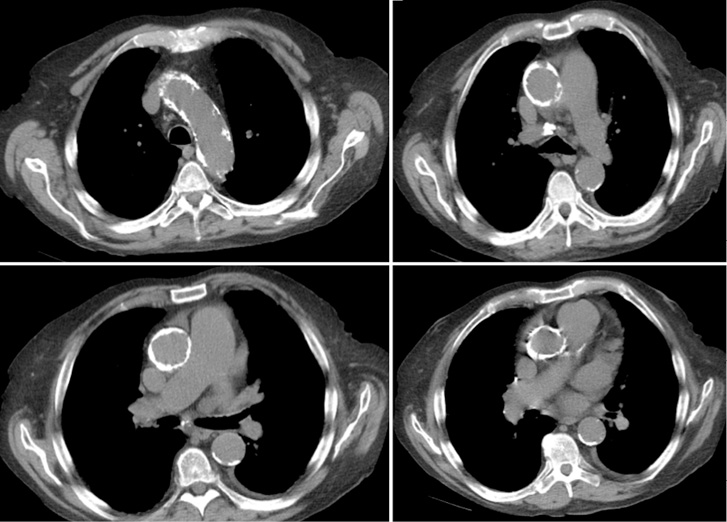
Figure 1. Porcelain aorta (or severely atherosclerotic aorta).
Figure 2. Hostile chest.
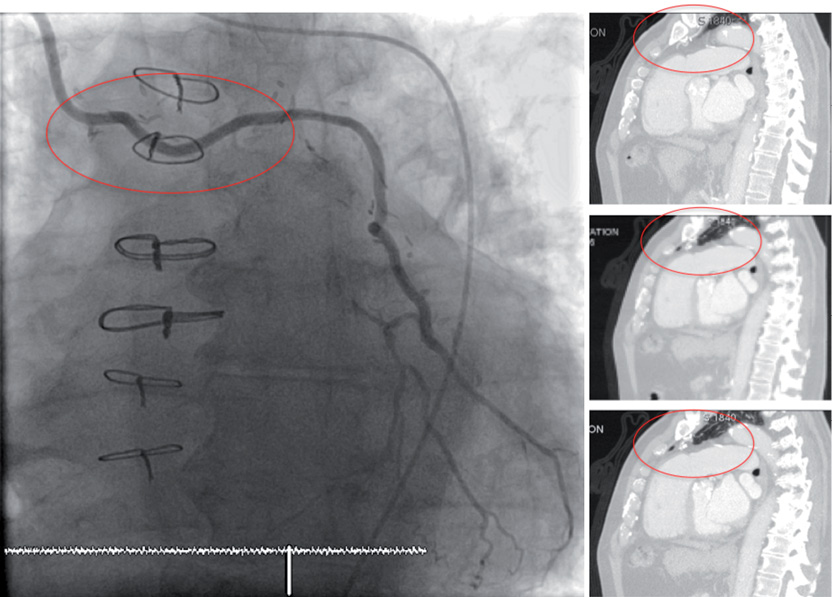
Figure 3. Patent IMA graft crossing midline and/or adherent to posterior table of sternum.
Patient stratification - The heart team approach
Valve Academic Research Consortium-2 recommends the use of a heart team for patient evaluation. The heart team should consist of at least (interventional) cardiologists, cardiovascular surgeons, and imaging specialists, but its composition is dynamic and can also include anaesthesiologists, geriatricians, neurologists, etc. This multi-disciplinary team should convene as a group on a regular basis to review and interpret clinical data to arrive at a consensus on the optimal treatment strategy for each patient. The heart team approach also allows for adjustment of the decision-making process according to local experience and circumstances.
The heart team should agree on an estimated 30-day mortality risk for each patient based upon integrating a careful clinical assessment and utilizing appropriate risk prediction scoring systems, preferably the STS score. Surgical mortality risk strata are difficult to precisely assign, but an estimated 30-day-mortality of <4% is considered low risk, 4-10% is intermediate risk, >10% is high risk, and >15% is very high risk. A patient is considered extreme risk if at least 2 cardiovascular surgeons from a tertiary centre of excellence deny surgery because of prohibitive operative risks, estimated to be a combined >50% risk of irreversible morbidity or mortality13. In addition to the specific risk factors that can prohibit patients from undergoing TAVI or surgical aortic valve replacement (SAVR) (Table 1), operative risk assessment is also important to identify patients who are likely not to benefit from either TAVI or SAVR (the so-called “futility” category of high-risk patients). An expected improvement in quality of life may further be necessary to identify treatment responders versus non-responders. Individualized life expectancy assumptions should be incorporated by the heart team in the clinical decision-making process as a central factor in weighing the risk-benefit ratio. Prognostic indices of life expectancy may play a central role in moving beyond arbitrary age-based cutoffs14.
The most important role of the heart team is to provide customized management decisions for common and unusual clinical scenarios in terms of patient selection, procedural performance and complication management. An example is the frequent situation of severe AS and concomitant coronary artery disease (CAD). The complexity of CAD and appropriate revascularization strategies in the setting of AS should be determined by consensus from interventional cardiologists and cardiovascular surgeons15,16. In new TAVI clinical trials, angiographic risk scores (e.g., SYNTAX score) may be utilized to help determine the complexity of CAD, as a basis for inclusion in the trial. Thresholds for coronary revascularization and the choice for a staged or concomitant PCI with TAVI should be guided by the complexity of the CAD and other factors as determined by the heart team17,18. In general, the plan to deal with other coexisting conditions (such as atrial fibrillation, other valvular lesions, and other congenital lesions) should be prespecified and all complications encountered in the treatment of associated conditions (including treatment after the TAVI procedure) should be captured. Such thorough pre-procedural assessment is also valuable in discriminating new post-procedural complications from simple exacerbations of pre-existing conditions.
Clinical endpoints
MORTALITY
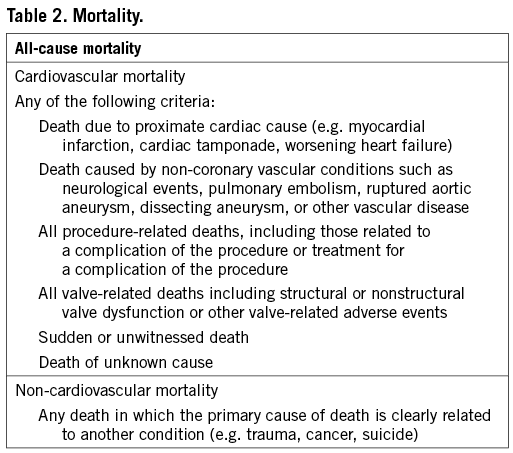
In addition to the original VARC definitions, VARC-2 recommends collection of immediate procedural mortality to capture intra-procedural events that result in immediate or consequent death ≤72 h post-procedure. Taking into account the surgical literature, procedural mortality consist of all-cause mortality within 30 days or during index procedure hospitalization –if the postoperative length of stay is longer than 30 days.
The cause of death should be captured, based on a careful review of narrative summaries and source materials. All-cause, cardiovascular, and non-cardiovascular mortality should be reported after 30 days during follow-up (Table 2). In determining the cause of death, the adjudication committee should consider the clinical context at the time of the index procedure and during the time interval leading up to death. All efforts (including use of national death registries) should be made to identify, precisely characterize, and appropriately classify any death.
MYOCARDIAL INFARCTION
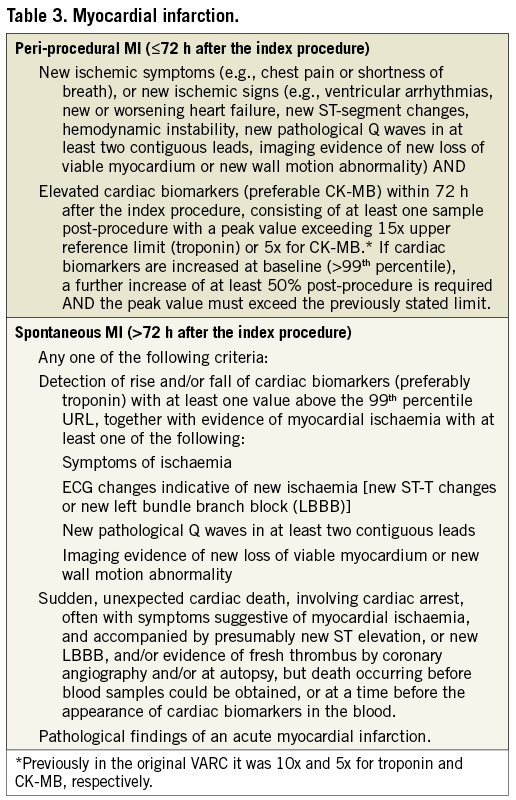
Myocardial injury as determined by a significant rise in cardiac biomarkers occurs frequently following TAVI, and a significant magnitude of myocardial injury has been associated with worse outcomes19. Valve Academic Research Consortium-2 recommends systematic collection of biomarkers of myocardial injury prior to the procedure, within 12-24 h after the procedure, at 24 h thereafter, at 72 h or at discharge, and if still elevated daily until values are declining. Similar to the previous VARC recommendations, the definition of peri-procedural (≤72 h following TAVI) MI will be based on a combination of clinical criteria and cardiac biomarkers. However, the threshold values have been adjusted (Table 3). Acute ischaemic events occurring after 72 h should be considered spontaneous myocardial infarctions and defined in accordance with the universal MI guidelines20.
STROKE
With increasing attention to stroke as an important peri-procedural complication of TAVI21, the FDA has increasingly emphasized the need for accurate assessment of stroke and has participated actively in recommending specific details of the VARC-2 definitions. In an attempt to further align with the fundamental definitions now endorsed by the FDA22, consensus was reached at VARC-2 to further refine the definition of stroke and recommend the use of these definitions in future TAVI clinical trials (Table 4). The definitions endorsed by the FDA are intended to apply to a wide range of clinical trials and to enable those trials to assess the clinically relevant consequences of vascular brain injury for determining the safety or effectiveness of an intervention.
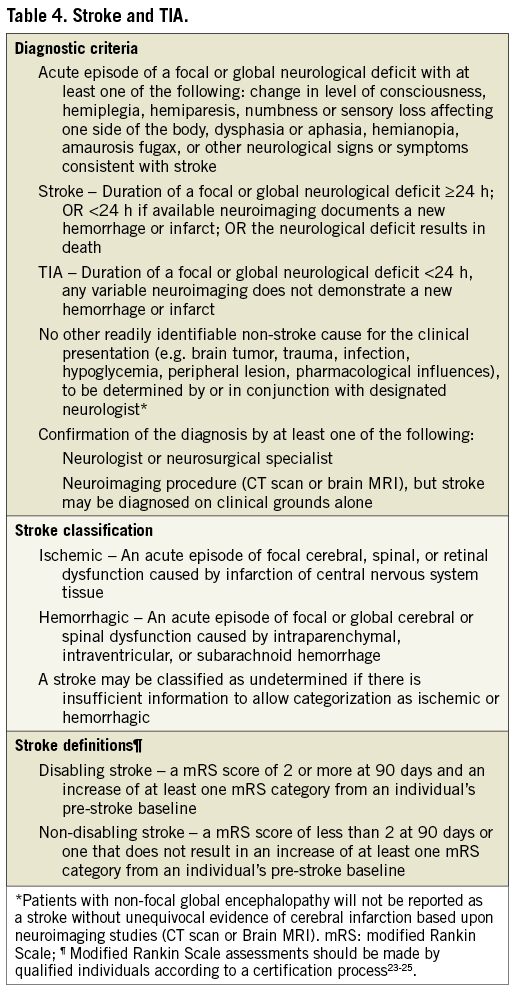
Stroke is defined as an acute episode of focal or global neurological dysfunction caused by brain, spinal cord, or retinal vascular injury as a result of hemorrhage or infarction. Stroke may be classified as ischemic or hemorrhagic with appropriate sub-definitions. Ischemic stroke is defined as an acute episode of focal cerebral, spinal, or retinal dysfunction caused by infarction of central nervous system tissue. Hemorrhagic stroke is defined as an acute episode of focal or global cerebral or spinal dysfunction caused by intraparenchymal, intraventricular, or subarachnoid hemorrhage. A stroke may be classified as “undetermined” if there is insufficient information to allow categorization as ischemic or hemorrhagic.
An entity closely related to ischemic stroke that should be assessed is transient ischemic attack (TIA). TIA is defined as a transient episode of focal neurological dysfunction caused by brain, spinal cord, or retinal ischemia, without acute infarction. The difference between TIA and ischemic stroke is the presence of tissue damage on neuro-imaging studies or new sensory-motor deficit persisting >24 hours. By definition, TIA does not produce lasting disability.
Valve Academic Research Consortium-2 recognizes that an assessment of stroke is incomplete without an appropriate measurement of the disability resulting from the stroke. VARC-2 recommends the use of the modified Rankin Scale (mRS) to assess this clinical disability23-25. Assessment of the mRS should occur at all scheduled visits in a trial and at 90 days after the onset of any stroke. This approach will maximize the detection of new or recurrent strokes, assist in ongoing evaluation of events previously determined as TIA, and provide an accepted and reliable indicator of the long-term impact of a given stroke.
Previously, Valve Academic Research Consortium-2 recommended categorizing strokes as “major” and “minor” based upon mRS scores. In order to enhance accuracy in the description of a given stroke and to provide accurate categorization of strokes within a given trial, Valve Academic Research Consortium-2 now recommends the use of the terms “disabling” and “non-disabling”. A disabling stroke is one that results (at 90 days after stroke onset) in a mRS score of 2 or more and an increase of at least one mRS category from an individual’s pre-stroke baseline. A non-disabling stroke is one that results (at 90 days after stroke onset) in a mRS score of less than 2 or that does not result in an increase of at least one mRS category from an individual’s pre-stroke baseline. In addition to this categorization in disabling and non-disabling stroke, the endpoint of all stroke should be reported.
Although brain imaging (typically, MRI for acute and chronic ischemia and hemorrhage, and CT for acute and chronic hemorrhage and chronic ischemia) is often used to supplement the clinical diagnosis of stroke26, a diagnosis of stroke may be made on clinical grounds alone. Valve Academic Research Consortium-2 recognizes that stroke symptoms are protean and not well-suited to a pre-specified itemized listing. Accordingly, Valve Academic Research Consortium-2 recommends that a vascular neurologist experienced in clinical trials involving stroke be included in all phases of trial planning, execution, and monitoring, including involvement in the Clinical Events Committee and the Data and Safety Monitoring Board.
New insights into the timing of events show delayed or late occurrence of strokes, beyond the early post-implantation phase27. This may suggest that the cause of stroke is additionally related to other factors or patient susceptibilities and should generate active investigation of devices and adjunctive pharmacotherapy to reduce the frequency and severity of strokes after TAVI, including precise documentation of the use and dosage of antithrombotic and antiplatelet medication. Patient baseline characteristics (e.g., carotid stenosis) and postoperative complications (e.g., atrial fibrillation) need to be carefully documented to be able to identify contributing causes of stroke.
Invasive stroke management (catheter-based intracranial intervention) is gaining an increasingly important role and may impact morbidity and mortality. Valve Academic Research Consortium-2 therefore recommends ascertainment of any acute stroke management strategies (e.g., aspiration, thrombolysis, or conservative management).
BLEEDING COMPLICATIONS
Valve Academic Research Consortium-2 acknowledges the fact that the Bleeding Academic Research Consortium (BARC) recently convened and established standardized bleeding definitions for patients receiving antithrombotic therapy and undergoing coronary revascularization (PCI or CABG)28,29. However, because the current definitions have been well adopted and shown to be accurate in predicting adverse events30, Valve Academic Research Consortium-2 has chosen to maintain the original VARC definitions (Table 5), recognizing that future validation of BARC criteria in this population may warrant revision of the current recommendations.
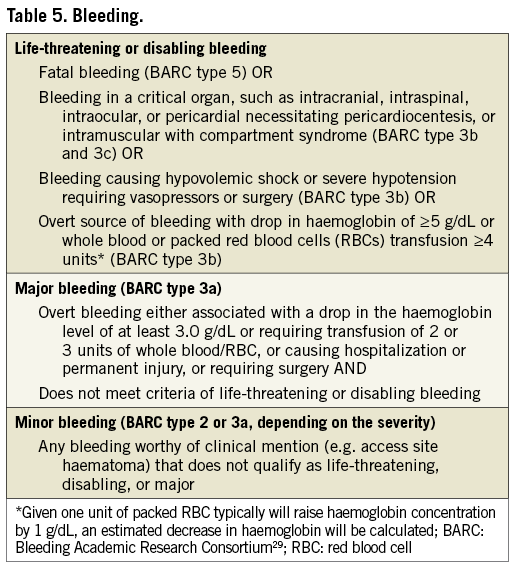
With respect to blood transfusions, it is critical to acknowledge that a bleeding complication has to be the result of overt bleeding and cannot be adjudicated based on blood transfusions alone.
ACUTE KIDNEY INJURY (AKI)
The original VARC definitions recommended use of a modified version of the RIFLE classification. However, we now recommend using the AKIN system (Table 6), which is a modified version of RIFLE that has been adopted by many in the nephrology community, including the KDIGO initiative31,32. As a result, AKI can also be diagnosed according to urine output measures (Table 6).
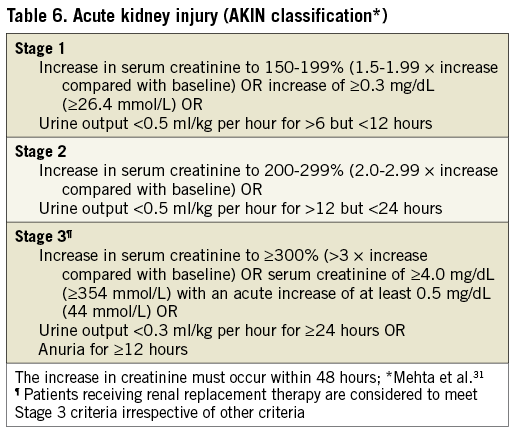
In comparison with the original VARC, the timing for diagnosis of AKI is extended from 72 h to 7 days. Patients that experience AKI should have follow-up renal function assessments after 7 days until stabilization.
VASCULAR COMPLICATIONS
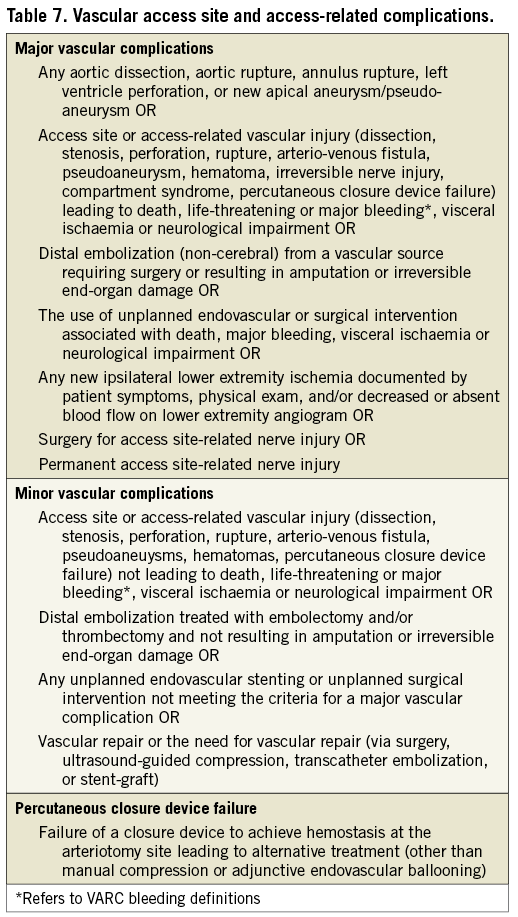
Table 7 lists VARC-2 definitions for major and minor vascular complications. Further clarifications of these definitions to supplement the original VARC document are as follows. Pre-planned surgical access or a planned endovascular approach to vascular closure (e.g., “pre-closure”)33,34 should be considered as part of the TAVI procedure and not as a complication, unless untoward clinical consequences are documented (e.g., bleeding complications, limb ischaemia, distal embolization, or neurological impairment). Unplanned endovascular stenting or surgical repair for any vascular complications during the index procedure without other clinical sequellae should be considered as a minor vascular complication, except if associated with qualifying consequences (Table 7). Complications related to alternative access sites, including the left-ventricular apex, subclavian artery, or aorta should be systematically recorded. In order to ensure accurate capture of these elements, VARC strongly recommends that detailed information regarding the access site and pre-planned vascular closure technique be recorded as well as the use of any additional unplanned access or closure techniques (surgical repair, endovascular stenting or endovascular balloon therapy). Since many vascular complications will also result in a bleeding complication, events that meet VARC-2 definitions for both categories should be reported in both categories. Finally, VARC-2 recommends that all vascular complications be recorded as either access (e.g., iliac rupture) or non-access site related (e.g., ascending aorta dissection or rupture unless aortic access is used and the event originates from cannulation site).
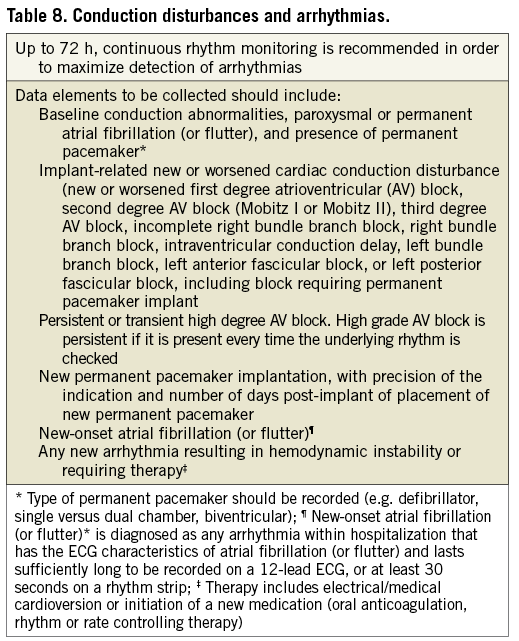
CONDUCTION DISTURBANCES AND ARRHYTHMIAS
Valve Academic Research Consortium-2 proposes systematic collection of data on the frequency of implant-related new and/or worsened conduction disturbances and the incidence and indication for permanent pacemaker implantation (Table 8). In addition, the frequency of specific arrhythmias following TAVI should be recorded as they may result in prolonged hospitalization and impaired clinical outcomes. New-onset atrial fibrillation (or flutter) is diagnosed as any arrhythmia within hospitalization that has the ECG characteristics of atrial fibrillation (AF) and lasts sufficiently long to be recorded on a 12-lead ECG, or for at least 30 seconds on a rhythm strip35. The therapeutic approach to new-onset AF (spontaneous conversion, electrical or medical cardioversion, initiation of oral anticoagulation, and rate or rhythm control medications) and any clinical consequences should be thoroughly documented in the case report form.
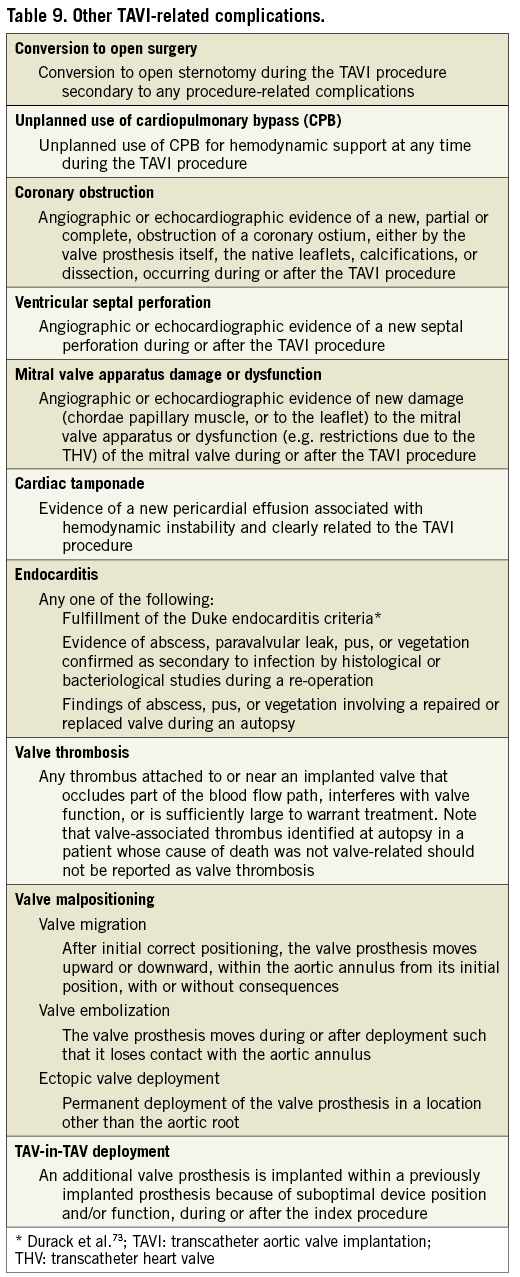
OTHER TAVI-RELATED COMPLICATIONS
The original VARC document recommended collection of a number of TAVI-related complications, but did not provide specific endpoint definitions for several endpoints. Valve Academic Research Consortium-2 recommends reporting any other complications related to the TAVI procedure, even those occurring less frequently, and provides formal Valve Academic Research Consortium-2 definitions (Table 9)36-38.
ADDITIONAL CONSIDERATIONS
For studies or trials where the occurrence, prevention or treatment of cerebral infarction is a fundamental feature (e.g., embolic protection devices) additional appropriate imaging in all or a subset of patients may be necessary to allow determination of effectiveness.
Valvular function
VARC-2 maintains the original recommendations to use echocardiography as the primary imaging modality for assessment of prosthetic valve function39. This should include valve position, morphology, function, and evaluation of LV and RV size and function. The suggested time-points for routine follow-up transthoracic echocardiography (TTE) following valve implantation are: immediately (before discharge) following implantation for transarterial approaches or within 30 days for transapical or transaortic approaches, 6 months following implantation, 1 year following implantation, and yearly thereafter. At these endpoints, prosthetic aortic valve stenosis and regurgitation should be reported.
TRANSCATHETER VALVE STENOSIS
The assessment of prosthetic valve stenosis should be an integrative process utilizing multiple parameters of valve function. Table 10 outlines the primary parameters used for assessing prosthetic valve function based on published guidelines40. Divergence from the guidelines is based on a number of studies41,42 as well as methods used in large randomized control trials of TAVI43,44. In addition, VARC-2 does not recommend using acceleration time, which is dependent on ventricular function and heart rate42. The limitation of flow-dependent parameters such as peak jet velocity or mean transprosthetic gradient is obvious, however, even flow-independent parameters such as effective orifice area (EOA) and Doppler velocity index (DVI) have limitations: i) the absolute EOA does not account for the cardiac output requirements in relation to patient’s body size thus lower criteria should be used to define prosthetic valve stenosis in patients with BSA<1.6 m2 (Table 10), ii) the indexed EOA may overestimate the valve-related hemodynamic burden in obesity, hence, lower criteria may be more appropriate in patients with body mass index ≥30 kg/m2, iii) DVI severity criteria are dependent on left ventricular outflow tract (LVOT) size, thus a lower threshold may be more appropriate in patients with LVOT diameters of >25 mm. The EOA should generally be calculated with the use of the LVOT diameter and velocity measured just underneath the apical margin of the valve stent45,46. In cases where the landing zone of the stent is low in the LVOT, the diameter and velocity may both be measured in the proximal portion of the stent. Unlike the surgically-implanted valve, the transcatheter prosthetic valve EOA is defined not only by the size of the valve but also by the patient’s aortic valve/annular anatomy and procedural variables. Thus, well-established normal transcatheter valve gradients and EOAs based on pre-implant aortic annular dimensions do not currently exist. Clinicians should be aware of this variability when assessing a patients for transcatheter valve function and VARC-2 strongly recommends that the patient’s own initial post-implant study be used as a reference for serial comparisons.
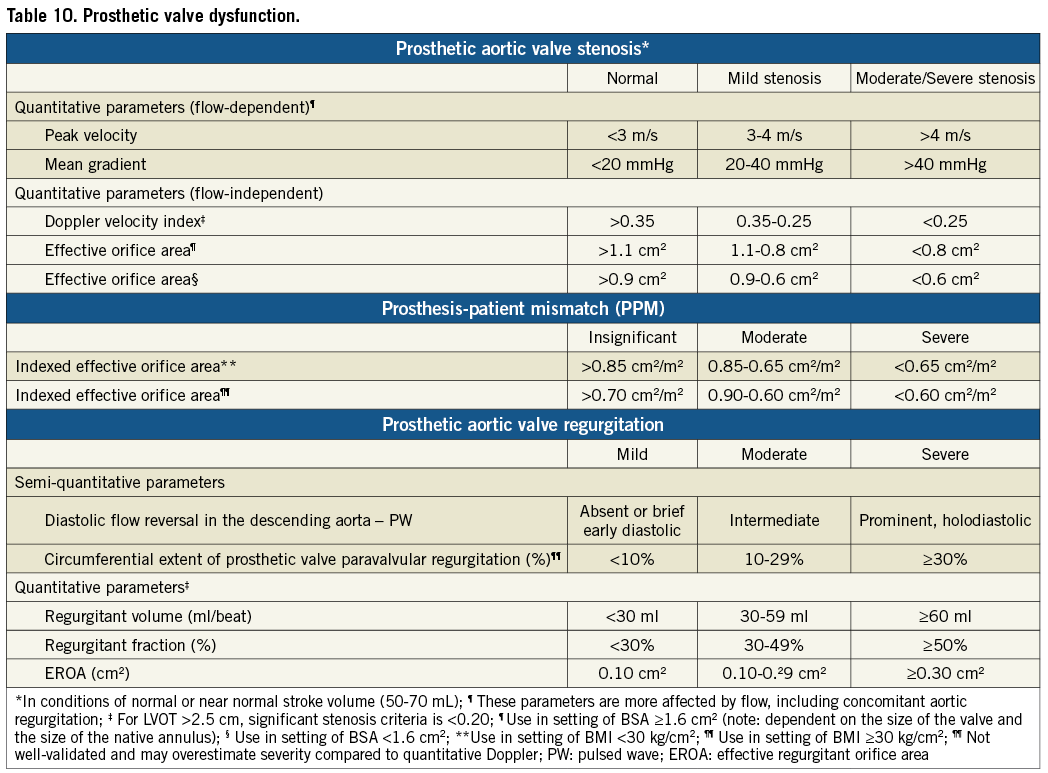
Assessment of transcatheter valve dysfunction includes the immediate post-TAVI hemodynamics and the follow-up evaluation. The immediate post-TAVI evaluation documents initial valve appearance (position and circularity of the stent, and leaflet morphology and motion) and a comprehensive hemodynamic evaluation. VARC-2 advocates using the integrative approach outlined in the algorithm shown in Figure 4 as part of a comprehensive hemodynamic evaluation by initially using one flow dependent (e.g., mean gradient) and one flow independent criterion (e.g., EOA) for the initial hemodynamic evaluation. If there is discordance between these measurements, then the DVI should be calculated. An abnormal DVI indicates possible prosthetic valve dysfunction. A normal DVI indicates intrinsically normal prosthetic valve function, and the indexed EOA can then be used to determine the reason for initial measurement discordance. When the indexed EOA is low in the setting of normal DVI, the patient probably has prosthesis-patient mismatch (PPM), an indicator of the intrinsic relationship of the implanted valve to the cardiac output requirements of the patient47. PPM occurs in the setting of a morphologically-normal valve and is considered to be hemodynamically insignificant if the indexed EOA is >0.85 cm2/m2, moderate if between 0.65 and 0.85 cm2/m2, and severe if <0.65 cm2/m2. However, for obese patients (body mass index ≥30 kg/m2) lower criteria may be more appropriate (Table 10).
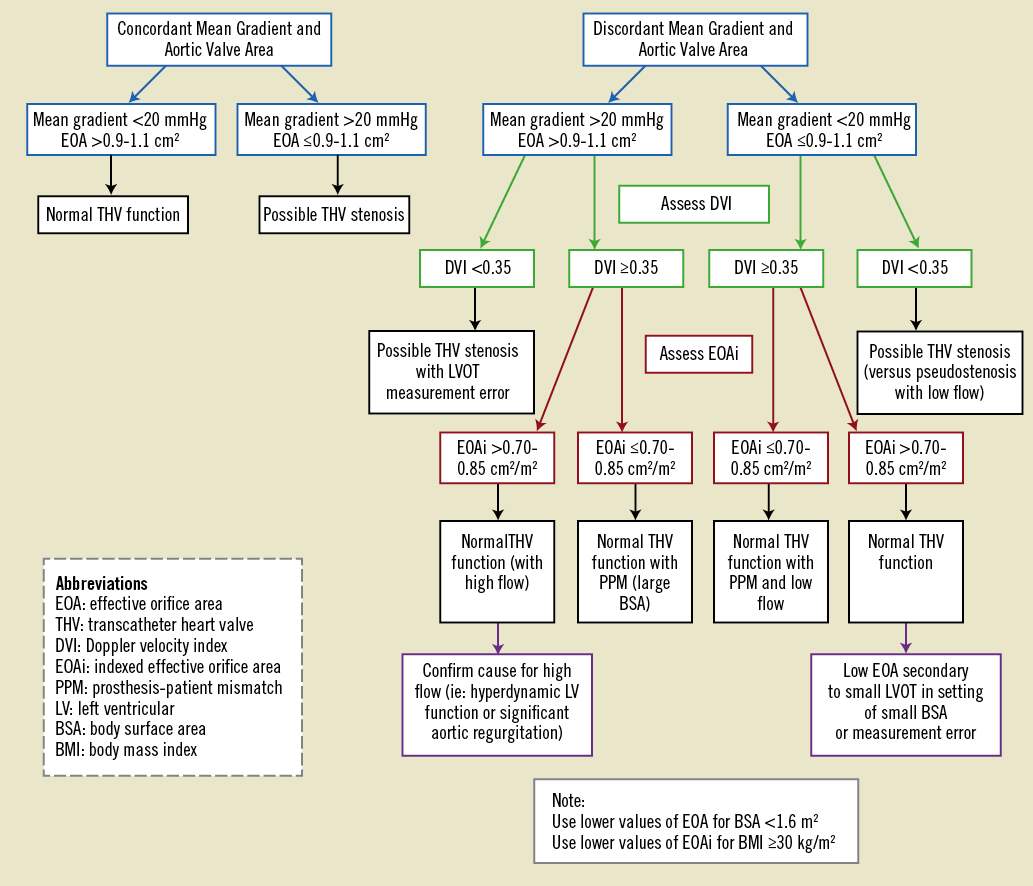
Figure 4. Transcatheter heart valve hemodynamic evaluation algorithm.
TRANSCATHETER VALVE REGURGITATION
There is growing evidence suggesting a significant association of post-procedural paravalvular regurgitation with short- and long-term mortality48,49. As the duration of implanted transcatheter heart valves increases, valve durability and dysfunction becomes more crucial. Evaluating the presence and severity of regurgitation should include an assessment of both central and paravalvular components, with a combined measurement of ‘total’ aortic regurgitation (AR) reflecting the summed volume load imposed on the LV (Table 10). Quantitative and semi-quantitative hemodynamic assessment of AR severity should be performed with Doppler echocardiography according to the guidelines39,50,51. Color Doppler evaluation should be performed just below the valve stent for paravalvular jets, and at the coaptation point of the leaflets for central regurgitation. Although all imaging windows should be used, the parasternal short-axis view is critical in assessing the number and severity of paravalvular jets. Whenever possible, quantification of prosthetic regurgitant volume, effective regurgitant orifice area and regurgitant fraction (Table 10) should be performed40,51,52. The regurgitant volume may be calculated as the difference between the stroke volume across any non-regurgitant orifice (RVOT or mitral valve) and the stroke volume across the LVOT.
It is important to realize that at this time the body of evidence supporting the numerical criteria used in Table 10 as well as Figure 4 may be limited. These criteria should be used as guidelines for clinical decision-making and require further validation as our experience continues to expand.
FOLLOW-UP ASSESSMENTS
The follow-up assessment should also begin with valve imaging and documentation of changes in morphology. When determining whether a patient has developed hemodynamically significant structural valve failure the patient’s own baseline echocardiographic parameters should be used as a reference. An increase in mean gradient >10 mmHg, a decrease in EOA >0.3-0.4 cm2 or a reduction in DVI >0.1-0.13 probably indicates a change in valve function and should trigger a comprehensive hemodynamic evaluation. Whenever valve dysfunction is suspected, careful evaluation of valve morphology should confirm a structurally abnormal valve. In addition, measurement error must be excluded; use of a consistent LVOT diameter for more accurate follow-up study comparisons is recommended. Finally, changes in ventricular morphology would be expected in the setting of long-standing significant valvular dysfunction and this parameter may support the clinical assessment of severity.
Although the rate of moderate or severe regurgitation may appear to be less at follow-up, this may be the result of attrition of the sickest patients. To assess such time-trends it is recommended to report individual patients’ progression of regurgitation, in a table that provides changes between short-term and long-term regurgitation, including mortality48.
Quality of life
QUALITY OF LIFE EVALUATION IN AORTIC STENOSIS
New York Heart Association (NYHA) classification is limited by the discrete nature of the scale, which provides only modest resolution to detect clinically relevant changes. Moreover, since NYHA class is assessed by an external body rather than the patient, it does not reflect the patient’s perspective. Thus, NYHA class is more properly considered a measure of functional status than quality of life (QOL).
The Minnesota Living with Heart Failure Questionnaire (MLHF)53 and the Kansas City Cardiomyopathy Questionnaire (KCCQ)54,55 have a number of desirable properties for the evaluation of health-related QOL (HRQOL) in the setting of AS. Both instruments produce outcomes on a continuous scale, which improves responsiveness and sensitivity. Although only the MLHF has been specifically validated in patients with aortic valve disease56, preliminary experience with the KCCQ in patients undergoing TAVI has also demonstrated a high degree of responsiveness and internal consistency57.
RECOMMENDED ENDPOINTS AND TIMING OF ASSESSMENT
VARC-2 recommends that a comprehensive assessment of HRQOL for patients undergoing TAVI incorporate both a heart-failure specific measure (such as the KCCQ or MLHF) as well as one or more generic measures (such as the Medical Outcomes Study Short-Form 36 (SF-36), the Short-Form 12 (SF-12), or the EuroQOL (EQ-5D)58-60. The disease-specific measures offer improved sensitivity/responsiveness as well as clinical interpretability, whereas the inclusion of a generic health status measure is useful because it captures some additional domains. Furthermore, generic measures can enhance comparability across different diseases and populations and can be used to compare patients with population-level benchmarks.
For comparison of TAVI versus SAVR (or for comparison of alternative access sites for TAVI), we recommend that early QOL assessment be performed at 2 weeks, 1 month, and 3 months using a combination of generic instruments and pain scales (e.g. visual analog scale) to assess the early recovery process. Evaluation of QOL at an intermediate time point (e.g., 6 months) could also be considered in order to confirm that QOL recovery is complete by this stage. At later time-points (1-5 years), use of heart-failure specific instruments to identify the consequences of long-term valve performance may be more useful. Finally, the assessment of cognitive function at later time-points (1-5 years) may be valuable for comparison of surgical vs. catheter-based techniques, although these endpoints generally require highly-specialized and demanding neuropsychiatric testing61. In contrast, for comparison of alternative TAVI systems (as may be expected in the near future), HRQOL assessment should focus mainly on heart-failure specific endpoints at intermediate and later time-points (1-5 years), wherein between-device differences in hemodynamic performance or structural valve deterioration may emerge. Inclusion of disease-specific QOL measures in these studies can also provide insight into the consequences of valve-related complications such as the need for pacemaker insertion.
ADDITIONAL CONSIDERATIONS
It is essential to ensure complete ascertainment of HRQOL at each time-point, as missing data cannot be retrieved retrospectively and statistical adjustment techniques (e.g., multiple imputation) that assume that data are “missing at random” may not be adequate. Differential mortality between 2 treatments may complicate the interpretation of QOL results since QOL may appear to “improve” over time even with an ineffective therapy simply because of attrition of the sickest patients. The use of categorical endpoints that characterize outcomes as favorable (e.g. survival AND improvement of QOL endpoints)57,62 or endpoints that integrate survival and QOL (e.g., quality-adjusted life expectancy), may provide more interpretable results. In such cases, reporting the outcomes in both ways (i.e., among the entire study cohort and separately among only the surviving patients) will provide the most complete description of the results.
Composite endpoints
RATIONALE AND CAVEATS
Comparisons of success, safety, and effectiveness with achievable study cohort sample sizes may at times require use of composite endpoints. However, it is important that composites contain components that have roughly similar impacts on the patient. A family of single endpoints tending in the same direction may, as a family of hypotheses, be statistically significant when individual endpoints are not.
Each post-procedural event has a different temporal risk profile (hazard function) modulated by different risk factors. Therefore, traditionally, evaluation of safety and efficacy of procedures has focused on in-hospital events (complications and morbidity), events within 30 days of the procedure, and “late” events.
SPECIFIC COMPOSITE ENDPOINTS
Assessment of TAVI, SAVR, and their alternatives or new devices should include device, procedure, and patient-oriented endpoints. These endpoints have been devised to be applicable to both TAVI and SAVR. Previous clinical trials have used all-cause mortality at one year as the primary clinical endpoint. Due to the emergence of stroke as an important clinical event, future trials should also require the composite of all-cause mortality and disabling stroke as a primary or secondary endpoint.
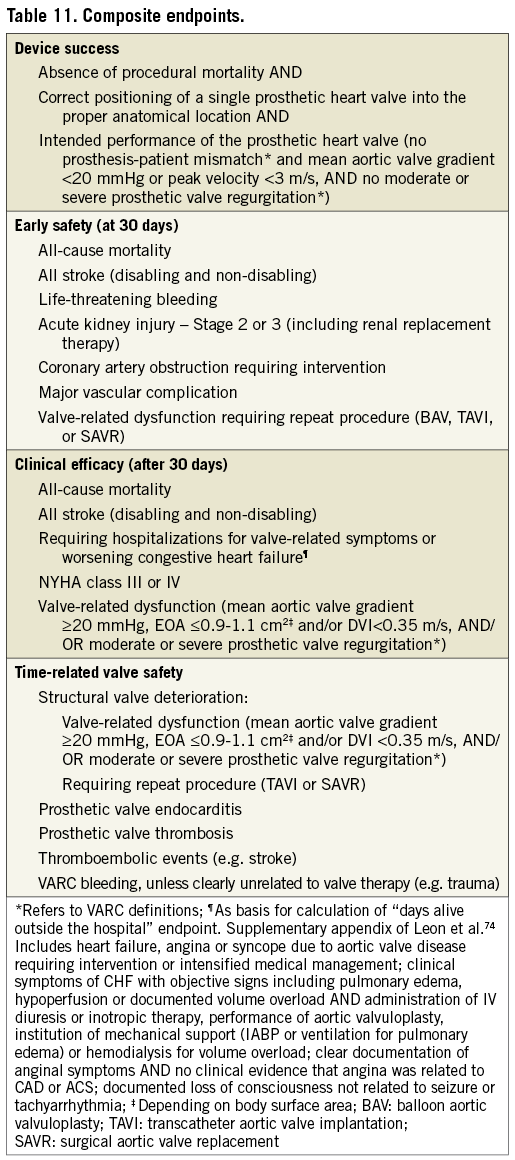
The first VARC document proposed three composite endpoints: device success, early safety, and clinical efficacy. VARC-2 goes beyond the early and intermediate experience of TAVI, drawing upon prior surgical AVR guidelines to include time-related safety endpoints63. Therefore, VARC-2 recommends a new composite endpoint, time-related valve safety, which combines valve dysfunction, endocarditis, and thrombotic complications of the prosthesis (Table 11).
Discussion
While the original VARC standardized endpoint definitions were fundamentally useful and have been widely adopted, growing experience with TAVI studies has identified some definitions as ambiguous, of limited clinical utility, or in need of updating or extension5,6,64,65. This need provided the rationale for a VARC-2 document with such improvements and additions. As was the case with the original VARC process, it should be emphasized that this consensus manuscript is not intended to be a guidelines document, but rather a practical tool to facilitate and inform clinical research in TAVI.
Current clinical trials are focusing more on intermediate risk patients, and more studies are comparing TAVI with surgical AVR. Therefore, it becomes increasingly important to identify those patients that benefit from either treatment. Specific risk categories have been defined to allow universal clinical study designs and outcome comparisons.
Changes and additions that have been applied to improve interpretation of clinical endpoint definitions and provide further insights on TAVI-related outcomes are: 1) Risk stratification should be done by a dedicated “heart team” and include other factors (e.g. frailty, porcelain aorta) beyond the traditional risk scores, and should take into account coexisting conditions; 2) immediate procedural death has been added to capture intra-procedural events that result in immediate or consequent death; 3) stroke ascertainment requires the use of precise definitions, standardized assessments, close collaboration with neurology experts including consideration of acute stroke management, and has been re-categorized as non-disabling or disabling; 4) detailed documentation of the etiology of strokes and concomitant therapies is needed to provide insights into the multi-factorial nature of acute, early, and late strokes; 5) closure device failure is now a separate category within vascular complications, and if unplanned percutaneous or surgical intervention does not lead to adverse outcomes, these are not considered as a major vascular complication, per se; 6) timing for AKI diagnosis has been extended from 72 h to 7 days; 7) AKI is diagnosed according to AKIN guidelines, which include classification by urine output to detect a wider range of etiologies; 8) peri-procedural myocardial infarction is defined by troponin or CK-MB elevation and has changed the troponin threshold from 10x ULN to 15X ULN based on recent data19; 9) assessment of conduction disturbances and arrhythmias has been reinforced66-69; 10) new definitions for several TAVI-related complications and valve malpositioning are reported; 11) echocardiography parameters of prosthetic valve stenosis and regurgitation have been updated and now include assessment of prosthesis-patient mismatch; 12) for quality of life assessment, VARC-2 recommends the use of both heart-failure specific and generic measures between 30 days and 5 years follow-up to fully assess the impact of the procedure and the durability of clinical benefit. These definitions can be used in studies comparing TAVI to surgical AVR, as well as in future trials comparing first generation to next generation TAVI devices.
The composite endpoint of device success has specifically been criticized for being too strict with regard to valve performance; for example, an AVA of >1.2 cm2 seems unachievable in patients with smaller body habitus5. The current VARC-2 definition therefore corrects for body surface area so that valve performance is now assessed through the indexed effective orifice area. It is notable that valve-in-valve procedures for failing bioprostheses will frequently have a low device success, even with this modified definition70. Considering that stroke in AS patients undergoing surgical or transcatheter AVR has emerged as an important concern, the composite of all-cause mortality and stroke should be considered as a primary or secondary endpoint in future trials. Two ongoing large randomized trials (PARTNER II [NCT01314313] and SURTAVI [NCT01586910]) are already incorporating these composite endpoints.
With longer follow-up duration, it becomes more critical to include time-related valve safety composite endpoints. This will eventually provide linearized rates of complications with transcatheter valves, known as ‘objective performance criteria’, as has been used to evaluate surgical valves71.
With this VARC-2 document we have provided further standardization of endpoint definitions and hope that adoption of these criteria will continue to increase, ultimately leading to improved comparability and interpretability of study results.
Funding
Grants have been provided to the ARC Board including representatives of Cardialysis, the Cardiovascular Research Foundation, Duke Clinical Research Institute, and Harvard Clinical Research Institute to cover costs of travel, meeting rooms, and lodging for academic attendees at the Washington and Rotterdam meetings by Abbott Vascular, Boston Scientific, Direct Flow Medical, Edwards Lifesciences, Heart Leaflet Technologies, Medtronic Corporation, and St. Jude Medical. Funding to pay the Open Access publication charges for this article was provided by Cardialysis BV on behalf of the Valve Academic Research Consortium.
Appendices
APPENDIX 1
VARC CONTRIBUTING GROUPS
1. Academic Research Organizations
Cardialysis (Rotterdam, The Netherlands)
Cardiovascular Research Foundation (New York, NY, USA)
Duke Clinical Research Institute (Durham, NC, USA)
Harvard Clinical Research Institute (Boston, MA, USA)
2. Societies
American College of Cardiology
European Association for CardioThoracic Surgery
European Society of Cardiology
Society of Thoracic Surgeons
3. US Food and Drug Administration
4. Industry Representatives
APPENDIX 2
VARC PARTICIPANTS
1. Clinical Research Organizations
1. Cardialysis/Erasmus MC - Rotterdam, The Netherlands
Head, SJ
Morel, MA
Serruys, PW
Van Es, GA
Van Mieghem, NM
Vranckx, P
2. Cardiovascular Research Foundation - New York, NY, USA
Généreux, P
Hahn, RT
Kirtane, AJ
Kodali, SK
Leon, MB
Maxwell, Y
Mehran, R
3. Duke Clinical Research Institute - Durham, NC, USA
Alexander, KP
Douglas, DS
Krucoff, MW
Petersen, J
4. Harvard Cardiovascular Research Institute - Boston, MA, USA
Cutlip, DE
2. Cardiologists
Borer, JS - Howard Gilman Institute for Heart Valve Diseases, Brooklyn, NY, USA
Cohen, DJ - Saint Luke’s Mid America Heart Institute, Kansas City, MO, USA
Holmes, DR Jr - Mayo Clinic Rochester, Rochester, MN, USA
Iung, B - CHU Bichat, Paris, France
Makkar, RR - Cedars-Sinai Heart Institute, Los Angeles, CA, USA
Piazza, N - German Heart Center, Munich, Germany; and McGill University Health Center, Montral, Canada
Popma, JJ - Beth Israel - Deaconess Medical Center, Boston, MA, USA
Rodès-Cabau, J - Quebec Heart & Lung Institute, Quebec, Canada
Thomas, M - Guys and St Thomas Hospital, London, UK
Tuzcu, EM - Cleveland Clinic Foundation, Cleveland, OH, USA
Vahanian, A - CHU Bichat, Paris, France
Webb, JG - St Paul’s Hospital, Vancouver, BC, Canada
Windecker, S - University Hospital Bern, Bern, Switzerland
3. Surgeons
Adams, DH - Mount Sinai Medical Center, New York, NY, USA
Cameron, DE - The Johns Hopkins Medical Institutions, Baltimore, MD, USA
Fontana, GP - Lenox Hill Heart and Vascular Institute, New York, NY, USA
Kappetein, AP - Erasmus MC, Rotterdam, the Netherlands
Mack, MJ - Baylor Health Care Systems, TX, USA
Maisano, F - San Raffaele Hospital, Milan, Italy
Miller, DC - Stanford University, CA, USA
Moat, NE - Royal Brompton and Harefield National Health Service (NHS) Foundation Trust, London, United Kingdom
Walther, T - Kerckhoff Heartcenter Bad Nauheim, Bad Nauheim, Germany
4. Echocardiographers
Geleijnse, ML - Erasmus University Medical Center, Rotterdam, The Netherlands
5. Neurologists
Brott, TG - Mayo Clinic, Jacksonville, FL, USA
Van der Worp, HB - University Medical Center Utrecht, Utrecht, The Netherlands
6. Statisticians
Blackstone, EH - Cleveland Clinic Foundation, Cleveland, OH, USA
7. US Food and Drug Administration
Aguel, F
Dunn, B
Getzoff, N
Laschinger, J
Patel, S
Sansing, V
Sastry, A
Swain, J
Zuckerman, B
8. Industry Representatives
Akin, J - Edwards Lifesciences, Orange, CA, USA
Allocco, D - Boston Scientific, Minneapolis, MN, USA
Armitage, T - Medtronic CardioVascular, Minneapolis, MN, USA
Bebeau, V - St. Jude Medical, Minneapolis, MN, USA
Concepcion, B - Boston Scientific, Minneapolis, MN, USA
Fonseca, T - Medtronic CardioVascular, Minneapolis, MN, USA
Robb, R - Medtronic CardioVascular, Minneapolis, MN, USA
Schroeder R - Heart Leaflet Technologies, Minneapolis, MN, USA
Sethuraman, B - Abbott Vascular, Santa Clara, CA, USA
Sheahan, B - Direct Flow Medical, Santa Rosa, CA, USA
Tatarek, N - St. Jude Medical, Minneapolis, MN, USA
9. Observers – Other
Fitzgerald, S
Carroll, JD
Edwards, FH
Lansky, AJ
Prager, RL
Conflict of interest
N. Piazza has received consultancy fees from Medtronic, his institution has received grants/grants pending from Medtronic. E.H. Blackstone has received study support from Edwards Lifesciences consultancy fees from Edwards Lifesciences. D.J. Cohen has received consultancy fees from Medtronic, his institution has received grants/grants pending from Medtronic and Edwards Lifesciences. G.A van Es is an employee of Cardialysis BV, The Netherlands. M.W. Krucoff has received study support from Edwards Lifesciences. S. Kodali is a Board member of St. Jude Medical and Thubrikar Aortic Valve and has received consultancy fees from Medtronic and Edwards Lifesciences. R. Mehran has received consultancy fees from Astra Zeneca, Janssen (Johnson & Johnson), Regado, Abbott Laboratories, Merck Sharpe & Dohme Corp., Maya Medical. Her institution has received grants/grants pending from BMS/Sanofi, The Medicines Company, Lilly/Daiichi Sanko. J. Rodés-Cabau has received consultancy fees from Edwards Lifesciences, St. Jude Medical. J.G. Webb has received consultancy fees from Edwards Lifesciences, his institution has received grants/grants pending from Edwards Lifesciences. S. Windecker has received speakers bureaus fees from Edwards Lifesciences and Medtronic, his institution has received a Swiss National Science Foundation Grant (32003B_135807) and has grants/grants pending from Edwards Lifesciences and Medtronic. M.B. Leon is on the advisory Board for Edwards Lifesciences. The others authors have declared to have no conflict of interests for this paper. The VARC meetings involved members of the Interventional Cardiology Devices Branch, of the Office of Device Evaluation, Center for Devices and Radiological Health, USFDA. The opinions or assertions herein are the private views of the authors and are not to be construed as reflecting the views of the FDA.

