- transcatheter aortic valve implantation
- aortic stenosis
- complications
Abstract
Aims: There is heterogeneity in the reporting of procedural outcomes and complications following transcatheter aortic valve replacement (TAVR). Recently, new definitions have been proposed by the Valve Academic Research Consortium (VARC) in an effort to standardise these outcomes and improve the quality of future clinical research. The aim of this study is to report the procedural outcomes and complication rates following TAVR in a large sequential patient cohort using a balloon expandable valve according to the new VARC definitions.
Methods and results: Three hundred and ten consecutive patients undergoing TAVR were assessed, including patients forming our early historical series at the infancy of TAVR. All complication rates were re-evaluated according to VARC definitions. Mean age was 82.2±8.1 years and the Society of Thoracic Surgeons score was 9.4±5.7%. Transfemoral 30-day mortality was 6.8% (3.8% in the second half of the cohort) and transapical 30-day mortality was 13.7% (9.4% in the second half of the cohort). Cardiovascular 30-day mortality was 7.4% and the composite safety endpoint at 30-days was 18.4%. Device success was 80% (post-procedural valve area ≤1.2cm2 in 9.7%). Failure to deliver and deploy a valve occurred in only 3.5%, with 82% (nine cases) occurring in the first half of the experience. Of those who did not meet echocardiographic criteria for device success (valve area ≤1.2cm2, transaortic gradient ≥20mmHg or ≥ moderate aortic incompetence), 90% achieved a New York Heart Association class I/II. Life threatening bleeding complications occurred in 8.4%. In 7.7% of patients, red blood cell transfusions were given without evidence of overt bleeding. Major strokes occurred in 2.3% and acute kidney injury occurred in 6.5%.
Conclusions: The VARC consensus guidelines provide a standardised reporting framework for clinical endpoints and complications post TAVR. We report the first series to our knowledge of 30-day outcomes using these definitions utilising a balloon expandable valve system.
Introduction
Transcatheter aortic valve replacement (TAVR) is a promising therapy for selected patients with severe aortic stenosis1-3. Despite procedural numbers growing rapidly worldwide, reporting clinical outcomes and associated complications has shown a lack of uniformity due to the absence of consistent definitions. Comparison of outcomes between trials and with device evolution is hence difficult.
Recently, the Valve Academic Research Consortium (VARC) has proposed standardised definitions for important clinical endpoints in TAVR in an effort to allow meaningful comparisons between clinical trials and to improve the quality of clinical research4. The VARC is an independent collaborative initiative utilising the combined expertise of various clinical specialties established in 2009.
We have previously reported outcomes and complications associated with TAVR at our centre, including procedural success rates, stroke rates, vascular complications and bleeding or transfusion needs2,5. The aim of the present study was to report for the first time on clinical endpoints and complication rates associated with implantation of a balloon expandable aortic valve according to the new VARC definitions, and to provide a framework of reference for other centres to compare their own outcomes according to the new VARC definitions.
Methods
Consecutive patients undergoing TAVR using the Edwards balloon-expandable valve (Edwards Lifesciences, Irvine, CA, USA) between January 2005 and April 2010 were included in the analysis. Patients in the randomised PARTNER trial were excluded from the cohort. The details of the transfemoral and transapical procedures were previously described6-9. All patients were approved on a humanitarian basis and gave written informed consent for the procedures. All patients were felt to have a prohibitive surgical risk as assessed by a team of senior cardiologists and cardiothoracic surgeons. All patients’ baseline characteristics, procedural details and clinical outcomes were entered into a dedicated database. All complications and TAVR specific endpoints were defined as per the VARC definitions4. In particular, the composite endpoint of “device success” is defined by VARC as: successful vascular access, delivery and deployment of the device with successful retrieval of the delivery system, with the device in the correct anatomical location and with an aortic valve area >1.2 cm2, mean aortic valve gradient <20 mmHg, without moderate or greater aortic regurgitation (AR), and with only one valve implanted in the correct anatomical location. All patients were assessed at hospital discharge and at 30-days. Mortality is reported as overall mortality and cardiovascular mortality. The 30-day combined safety endpoint is a hierarchical composite of all-cause mortality, major stroke, life-threatening bleeding, acute stage 3 kidney injury, periprocedural myocardial infarction, major vascular complication or repeat procedure for valve-related dysfunction. All patients with cerebrovascular events were reviewed and managed by a specialist neurologist and underwent cerebral imaging using computed tomography and if possible, magnetic resonance imaging. For patients in whom a modified Rankin score was not recorded prospectively at 30- and 90-days, detailed chart reviews were performed to estimate this as accurately as possible and classify strokes into major or minor events as needed.
Statistical methods
The data is presented using descriptive statistics. Continuous variables were tested for normality using the Shapiro-Wilks goodness of fit test; all were normally distributed hence presented as a mean with standard deviations. Categorical values are reported as absolute numbers and percentages. Comparison of categorical values was performed using a Chi square analysis or the Fisher’s exact test was applied if appropriate.
Results
Baseline characteristics
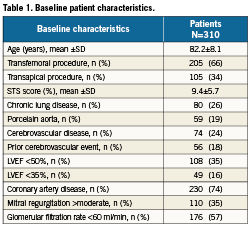
A total of 310 consecutive patients were included in the study. Of these, 205 had a transfemoral procedure and 105 had access via atransapical route (Table 1). Baseline risk was relatively high, with a mean Society of Thoracic Surgeons score of 9.4±5.7%, and mean age 82.2±8.1 years. Significant comorbidities were common, including chronic lung disease in 26%, porcelain aorta 19%, obesity 31%, prior cerebrovascular disease 24%, >moderate mitral regurgitation in 35% and glomerular filtration rate <60 ml/min in 57%.
Mortality
Overall 30-day mortality was 9.4%. There was improvement in overall mortality from the first to the second half of the cohort from 12.3% to 5.8% (p=0.02). Overall transfemoral 30-day mortality was 6.8%, with improvement from the first half of the cohort of 9.8% to 3.8% in the second half (p=0.06). Transapical 30-day mortality was 13.3%, improving from the first half of the cohort from 17.3% to 9.4% in the second half (p=0.11). Overall 30-day cardiovascular mortality was 7.4%, with 5.4% for the transfemoral group and 11.4% for the transapical cohort.
Device success
Overall device success according to the new VARC definitions was 80% (Table 2). Overall, 64 cases met at least one criteria inconsistent with device success. Of these 64, the aetiologies of “device failure” were as follows: post-procedural AVA of ≤1.2 cm2 (30 cases- 47%), post-procedural mean gradient ≥20 mmHg (9 cases- 14%), ≥moderate AR (17 cases- 27%), >1 valve implanted at the proper anatomical location (4 cases- 6%) and unsuccessful delivery and implantation of the device (11 cases- 17%). Importantly, no patients in the entire cohort met VARC criteria for significant stenosis post-procedure (AVA <0.8 cm2 or peak velocity >4 m/s). Of the patients with ≥2 AR, all had only 2+ AR (i.e., there were no cases of moderate-severe, or severe AR). The overall rate of clinical failure to implant a device was low: 3.5%, with the majority of cases occurring in our early experience (nine cases in the first half of the cohort and only two cases in the second half). Six patients had more than one aetiology of “device failure” e.g., AVA ≤1.2 cm2 and post-procedural mean gradient ≥20 mmHg.
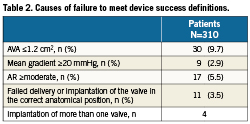
In the 30 cases with AVA ≤1.2cm2, all were women, with a median pre-procedural annulus diameter of 20 mm (IQR 19-21), and with the majority receiving 23 mm diameter valves (73%). Only four patients had SAPIEN XT valves, with four being early Cribier-Edwards valves and the remaining 22 being SAPIEN valves.
Patients who met haemodynamic criteria for failure of device success (post-procedural AVA of ≤1.2 m2, mean gradient ≤20mmHg, or ≥moderate AR) still had a dramatic improvement in functional class. At baseline, 88% were in NYHA class III or IV, and post procedure 90% were in NYHA class I or II. Of patients with post-procedural AVA ≤1.2 cm2, 97% were in NYHA class I or II.
Complications
Vascular and bleeding complications
A total of 17.4% of patients had a vascular complication, of which the majority were major complications (Tables 3 and 4). Of patients with major vascular complications, 84% had ≥4 units of packed red blood cells transfused.
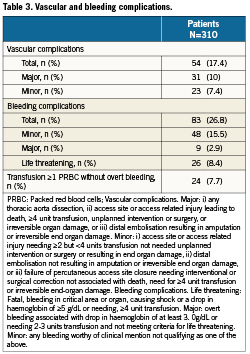
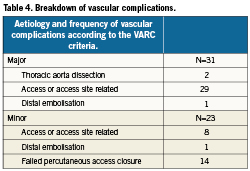
In the transfemoral cohort, 18.5% had vascular complications: major 9.8%, minor 8.8%. In the transapical cohort 15.2% had vascular complications: major 8.6%, minor 6.7% (Table 5).
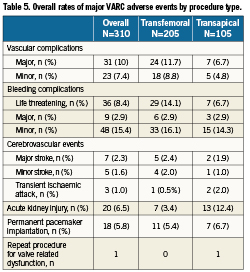
In total, 26.8% of patients had a bleeding complication. In 8.4% of patients, this met the criteria for life threatening bleeding, of which the majority (85%) resulted from vascular complications.
Of the total cohort, 7.7% underwent ≥1 PRBC transfusion with no overt source of bleeding.
Cerebrovascular complications
A total of 15 patients suffered a cerebrovascular complication (4.8%). The overall rate of major strokes was 2.3%, minor strokes 1.6% and transient ischaemic attacks in 1%.
Acute kidney injury
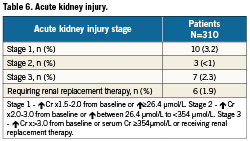
The total incidence of acute kidney injury was 6.5%, the majority of which (52.4%) was stage 1 (Table 6). There was only one case of stage 3 kidney injury in the transfemoral cohort, with the remainder in patients treated via the transapical route. Subsequent in-hospital mortality in patients requiring institution of haemodialysis was high –50%.
Repeat procedures for valve related dysfunction
One patient required balloon aortic valvuloplasty 3-days following valve implantation and no patients required valve re-operation in hospital or by 30-days.
Permanent pacemaker implantation
A total of 18 patients (5.8%) required implantation of a permanent pacemaker within 30-days.
Combined safety 30-day endpoint
The combined safety endpoint at 30-days was 18.4%.
Discussion
This study provides the first report, to our knowledge, of TAVR success and complication rates using a balloon expandable valve according to the recently proposed VARC definitions. Compared to earlier reports using individual site derived definitions, “device success” rates are lower and vascular and bleeding complications appear more frequent.
Device Success
The device success rate of 80% is lower than previously reported2-3,10-13. The reason for this lies in the definition; earlier reports focused largely on successful implantation of the device in the correct anatomical position without patient mortality, while the new definition proposed by VARC encompasses the latter as well as valve hemodynamic function. The main reasons for “device failure” were a calculated valve area ≤1.2cm2 in 9.7% of cases, followed by ≥moderate AR 5.5% (although AR was never more than moderate in any patient). Despite this, clinical and symptomatic improvement in these patients was dramatic, with 97% of patients with post procedural AVA ≤1.2 cm2 improving to NYHA class I or II. It is also reassuring that no patients met criteria for significant post procedural aortic stenosis (AVA<0.8 or peak transvalvular gradient of >4 m/s). Actual failure to deliver/implant the device occurred in only 3.5% of cases, with the vast majority of these occurring in the first half of this experience with first generation transcatheter valves and delivery systems.
Previous surgical series have documented mean post-procedural effective orifice area (EOA) as low as 1.1cm2 for some bioprosthetic valves, indexed EOA of 0.74 even for 23mm bioprostheses, and patient-prosthesis mismatch rates as high as 73%14-20. Transcatheter valves in the aortic position have shown comparable, if not better, hemodynamic function, larger overall EOAs and less patient prosthesis mismatch as compared to surgical bioprostheses21. We have recently shown that severe patient prosthesis mismatch occurs in approximately 5.7% of TAVR cases, comparing favourably with established surgical series22. However, the VARC criteria do not consider the patient’s body size or indexed AVA. In our cohort, all patients with AVA ≤1.2 were women and had a median annulus diameter of 20 mm, suggesting that indexed valve areas may be more relevant and representative.
Echocardiographic determination of AVA for transcatheter heart valves has not been standardised or validated, and methods of estimation vary. Clavel et al recently demonstrated that the EOA and incidence of prosthesis-patient mismatch vary significantly depending on whether the post TAVR left ventricular outflow tract diameter is measured at the insertion point of the prosthetic valve leaflets or immediately proximal to the prosthesis stent23. In that study, if the left ventricular outflow tract was measured at the insertion point of the leaflets, the average EOA was 1.29 vs. 1.60 cm² if measured just proximal to the stent frame (p<0.01). The incidence of severe prosthesis-patient mismatch was 2-fold different (p<0.01). This highlights the need for standardised echocardiographic evaluation protocols post TAVR to allow comparisons and consistent conclusions.
Measured valvular haemodynamics may also be affected by the clinical condition of patients in the post-operative period. In particular, conditions such as anaemia (which seem common given the transfusion rates) or fever may result in higher transvalvular gradients. The VARC definitions suggest that echocardiographic evaluation is performed ideally within 24-48 hours following TAVR. However, important patient clinical factors present at this early post-procedural period may affect the accuracy and conclusions of such tests, and it may be appropriate to delay this by a few days depending on the clinical scenario. It may be worth considering this in future revisions of the VARC recommendations.
A few other points regarding the present study are important to note. Our report captures an early historical cohort of patients, including the first series of transfemoral TAVR and the first off-pump transapical cases. We have previously shown that procedural success rates and clinical outcomes significantly improved since the early experience2. Only one size of valve was initially available, with the 26mm valve becoming available later in the experience, and more recently a 29mm valve. Knowledge concerning valve sizing (over sizing) was still in evolution and very early iterations of the delivery system were utilised, which did not have a tapered nose cone to aid in valve crossing. Subsequently, successful device delivery has approached 100% and valve over sizing has become routine2,5. We have also documented that patients generally have marked and maintained functional improvements following the procedure20,21.
Post-procedural AR is a common finding post TAVR, occurring in up to 80% of patients and generally being paravalvular and mild to moderate in severity2. Regurgitation does not appear to progress over time and does not seem to be associated with adverse haemodynamic or clinical sequelae to a follow-up as long as 5-years13. Nevertheless, TAVR thus far results in higher AR rates that surgical aortic valve replacement and longer-term follow-up is needed.
Vascular and bleeding complications
The occurrence of vascular complications according to the VARC criteria is higher than in previous reports from our centre2. Different centres have reported a range of vascular complications rates which depending on the definitions may be as high as 30%10,24-27. In the PARTNER B study TAVR was associated with major vascular complications in 16.2%28. We found, unsurprisingly, that the majority of patients with major vascular complications required ≥4 units of red blood cell transfusion. According to VARC, life-threatening bleeding is defined as requiring ≥4 units of red blood cell transfusion, and vascular access complications are the main causes of such bleeds. Nevertheless, some patients also had life threatening gastrointestinal bleeds. Patients also had vascular complications without bleeding – such as occluded iliofemoral vessels repaired either percutaneously or surgically.
The distinction between vascular complications in the setting of “failed percutaneous closure” may be a little difficult to ascertain. For example, a stenosis at the femoral access site that the operator dilates at the end of the procedure may arguably be considered a complication or alternatively just part of the percutaneous repair procedure. Iliofemoral balloon occlusion may be utilised routinely in some centres when removing large sheaths and securing percutaneous sutures while other centres may utilise this intervention only to tamponade in the management of failed haemostasis. This may lead to different reporting rates between operators who do vs. those who do not use such techniques.
We noted a significant proportion of patients undergoing blood transfusions without an obvious source of bleeding, and where anaemia was pre-existent or the cause of haemoglobin drop was unclear, such that this may warrant a possible separate category in future revised VARC criteria. The trigger for blood transfusions post-cardiac surgery is controversial. The current Society of Thoracic Surgeons and guidelines acknowledge that the benefits of blood transfusions have not been clearly demonstrated and that evidence is lacking to provide definitive recommendations29,30. They suggest avoiding transfusions in post cardiac surgical patients unless the haemoglobin is below 7g/dL (class IIa recommendation). However, they also suggest that it is “not unreasonable to transfuse red cells in certain patients with critical non-cardiac end-organ ischaemia whose haemoglobin levels are as high as 10 g/dL (class IIb recommendation), but more evidence to support this recommendation is required”. While a conservative approach to blood transfusions may be desirable, the possibility of ongoing or recurrent bleeding, non-revascularised coronary artery disease or other factors may lead to transfusion in the absence of major bleeding.
Cerebrovascular complications
The rates of overall cerebrovascular complications are encouraging and generally in keeping with previous reports of balloon expandable TAVR studies10. Previous reports have shown a range of cerebrovascular complications between 3-10%, possibly resulting from the variety of definitions used1,3,31. VARC has proposed clinically relevant stroke distinctions such that clinical assessments are also made after 30 and 90-days to evaluate events as major or minor strokes.
The determinants and outcomes of stroke post TAVR are poorly understood. We have previously reported that patients may be at increased risk of neurological events for up to 60 days post TAVR32. New cerebral MRI defects occur in up to 70% of patients post TAVR, with similar rates for transfemoral and transapical procedures33,34. However, there is a large discrepancy between these rates and documented clinical neurological events, and the significance of such MRI lesions remains unclear.
Acute renal injury
Previous studies have shown that acute renal injury post TAVR occurs in 12-28% of cases and is associated with a four-fold risk of post-operative mortality35,36. In our cohort, patients who required post-procedural renal replacement therapy had a 50% 30-day mortality. The majority of post-procedural renal impairment was stage1 and reversible.
Utility of VARC definitions
The VARC should be credited with addressing a pressing issue in reporting of outcomes and complications during TAVR. Given the rapid uptake of this technology standardisation of outcomes and future clinical research endpoints is paramount. TAVR operators should be encouraged to report TAVR outcomes according to the VARC criteria. Clarification of these collaborative criteria may be incorporated into future revisions (for example, indexed AVA or standardisation of left ventricular outflow tract measurement positions).
Limitations
As our data represents a single centre’s experience rather than the outcomes of a clinical trial, it is self-reported with no external independent data adjudication. Also, since our data is entered prospectively and relates to cases performed prior to the inception of the VARC definitions, re-evaluation of retrospective documentation relating to clinical events as they are classified according to the VARC criteria may result in underestimation of the true frequency of some events. It is possible that some minor complications may not have been captured, e.g., access site haematoma that does not qualify as life threatening, disabling or major is considered a minor complication.
Conclusions
The new VARC definitions provide an important tool to help standardise future TAVR clinical research endpoints and help compare clinical trials. We report for the first time to our knowledge the results of a series using the balloon expandable valves utilising these new criteria.
Conflict of interest statement
Drs. Webb, Cheung and Ye are consultants to Edwards Lifesciences.

