Abstract
Aims: TAVI is a minimally invasive alternative to surgical aortic valve replacement or medical therapy in patients with a high or prohibitive operative risk. The clinical significance of baseline anemia and prognostic implications in this patient cohort are unknown. We sought to evaluate the prevalence and prognostic implications of baseline anaemia in patients undergoing transcatheter aortic valve implantation (TAVI) at our institution.
Methods and results: One hundred and eighteen consecutive patients who underwent TAVI with the Medtronic Corevalve System (Medtronic Corp., Minneapolis, MN, USA) were included in the analysis. Clinical and biochemical data were prospectively collected before, during and after the procedure. Clinical follow-up was set at one month, one year and yearly thereafter. Anaemia was defined as a haemoglobin level <13 g/dL in men and <12 g/dL in women. Mortality was confirmed by consultation of the civil registry. The prevalence of baseline anaemia was 49%. Anaemic patients undergoing TAVI required more RBC transfusions (3.3±3.1 versus 1.5±2.3; p<0.001) and more frequently experienced a prolonged index hospitalisation exceeding two weeks. For patients with at least 1-year follow up (N=74), mortality at 30 days was no different; however 1-year mortality was significantly higher in the anaemic cohort (44 versus 15%, p=0.006). In a multivariable analysis, baseline anaemia emerged as an independent predictor of 1- year mortality (HR 2.10 [1.06-4.18]).
Conclusions: In our series, baseline anaemia is common in patients undergoing TAVI, forecasts a need for more red blood cell transfusions and is associated with increased 1-year mortality.
Introduction
Transcatheter aortic valve implantation (TAVI) is a less invasive alternative to surgical aortic valve replacement or medical treatment in symptomatic patients with severe aortic valve stenosis1,2. Currently this catheter based strategy is reserved for patients with a high or prohibitive operative risk3-7. Accordingly TAVI targets the elderly in whom a higher prevalence of baseline anaemia may be present. Baseline anaemia is associated with red blood cell (RBC) transfusions and in-hospital and late mortality in patients undergoing cardiac surgery and percutaneous coronary interventions (PCI)8-12. In patients undergoing TAVI, functional class, COPD and left ventricular dysfunction have been reported as predictors of procedural success and mortality3,13. Yet, the role of baseline anaemia has not been investigated. We, therefore, sought to investigate the prevalence of baseline anaemia and its prognostic effects on 30-day and 1-year mortality.
Methods
Patients and procedure
The study population consists of all consecutive patients with symptomatic severe aortic valve stenosis who underwent TAVI with the Medtronic CoreValve system (Medtronic Corp., Minneapolis, MN, USA) between November 2005 and February 2010 at the Thoraxcenter, Rotterdam, The Netherlands. Procedural details have been described before14. All TAVI procedures evolved under general anaesthesia. After TAVI the combination of aspirin and clopidogrel was recommended for three to six months. Patients on coumadin received triple therapy for one month after which aspirin was stopped. Clopidogrel was continued for a total of three months.
Definitions and data collection
Predefined baseline characteristics, medication and technical measurements (ECG, echo-Doppler) were prospectively collected during the outpatient clinic visit prior to the procedure. In addition, blood samples for haematology and biochemistry assessment were collected daily starting one day pre-treatment up to 72 hours post-treatment and included haemoglobin (Hb) concentration, white blood cell count, platelet count and creatinine.
Baseline anaemia was defined according to the World Health Organisation (WHO) as haemoglobin (Hb) level of <13 g/dL for men and <12 g/dL for women and was based on a blood sample one day before the TAVI procedure. Since packed red blood cell (RBC) transfusions influence the postprocedural Hb measurements, the modified Landefeld equation was used to estimate the corrected nadir haemoglobin level and the net haemoglobin drop post TAVI: assuming one unit of packed RBC represents 1 g/dL of Hb, the net blood loss corresponds to the addition of the number of packed RBC to the baseline-minus-measured nadir haemoglobin15,16. The corrected nadir haemoglobin level is then the baseline haemoglobin minus the net blood loss.
Net blood loss (in g/dL) = net Hb drop (in g/dL) = number of pRBC + (Baseline Hb – nadir Hb)
Corrected nadir Hb = Baseline Hb – net blood loss
The total amount of packed RBC transfused per patient periprocedural and during the entire hospitalisation was recorded by the institution’s blood bank. Thrombocytopenia was defined as a platelet count <140×109. The Valve Academic Research Consortium (VARC) has generated a consensus statement on TAVI related definitions (second VARC meeting, December 5-6, 2009, Amsterdam, The Netherlands). According to VARC acute kidney injury (AKI) was defined as an absolute increase in the highest value of serum creatinine ≥0.3 mg/dl (≥26.4 μmol/l), or a percentage increase in the highest value of serum creatinine ≥50% (1.5-fold from baseline) within 72 hours after TAVI. The definitions of vascular and bleeding complications as suggested by VARC have recently been summarised17,18.
RBC transfusion policy
The decision to start periprocedural RBC transfusion was the prerogative of the treating anaesthesiologist/intensivist on a case-by-case basis. RBC transfusions could be given during TAVI procedures while vascular or bleeding complications were evolving or at times of hemodynamic instability without awaiting haemoglobin measurements. Therefore, there was no predefined haemoglobin threshold to initiate RBC transfusions.
Follow-up
Clinical follow up information was collected during out-patient clinic visits after TAVI at one month, one year and yearly thereafter. Mortality was verified by the consultation of the Dutch civil registry.
Statistical analysis
Categorical variables are presented as frequencies and percentages and were compared with the chi-square or Fisher’s exact test. Normally and skewed distributed continuous variables are presented as means ± SD and medians (IQR), respectively. We used a Student’s or Wilcoxon’s rank-sum test for the comparison of continuous variables. A stepwise Cox multivariable regression analysis including all variables with p<0.10 in the Cox univariable analysis was used to determine the predictive factors of 1-year mortality. The Kaplan-Meier method was used to determine stratified survival rates for patients with and without baseline anaemia up to two years after TAVI. A log-rank test was used to compare the survival rates between these groups. A two-sided p<0.05 was considered to indicate significance. All statistical analyses were performed with SPSS version 15 software (SPSS Inc., Chicago, IL, USA).
Results
We included 118 patients who underwent TAVI at our institution. The transfemoral approach was the default strategy and applied in 115 cases. Surgical cut-down for trans-subclavian access was selected in three cases. Baseline characteristics and periprocedural and outcome data dichotomised for the presence of baseline anaemia are presented in Table 1-5. Forty-nine percent of patients were anaemic before the index procedure. All patients with anaemia underwent a focused work-up to detect reversible causes of anaemia (gastrointestinal evaluation, complete blood iron status, bone marrow analysis etc.) however in the majority of cases the anaemia was deemed multifactorial (minor nutritional deficiencies, lingering myelodysplastic syndromes, chronic kidney disease) for which no curative treatment option was available. There were no differences in baseline characteristics between the two groups (Table 1). Fifty-nine percent of the entire study cohort received at least one unit of packed RBC within 24 hours following TAVI, increasing to 70% during the entire hospitalisation. Patients with baseline anaemia reached lower postprocedural nadir haemoglobin levels and required significantly more RBC transfusions after TAVI in comparison to patients without baseline anaemia (Table 2). Forty and 17% of the anaemic patients received two or three and at least four units of RBC, respectively. The predominant reason for periprocedural RBC transfusion was femoral access site bleeding (Table 3). The net Hb-drop corrected for RBC transfusions was similar regardless of baseline Hb-level, implicating there was no excess blood loss in anaemic patients. Accordingly there was no difference in vascular or bleeding complications according to the VARC definitions (Table 4). More patients in the anaemic cohort had a length of in-hospital stay exceeding 14 days (38 versus 17%, p= 0.009) (Table 4).
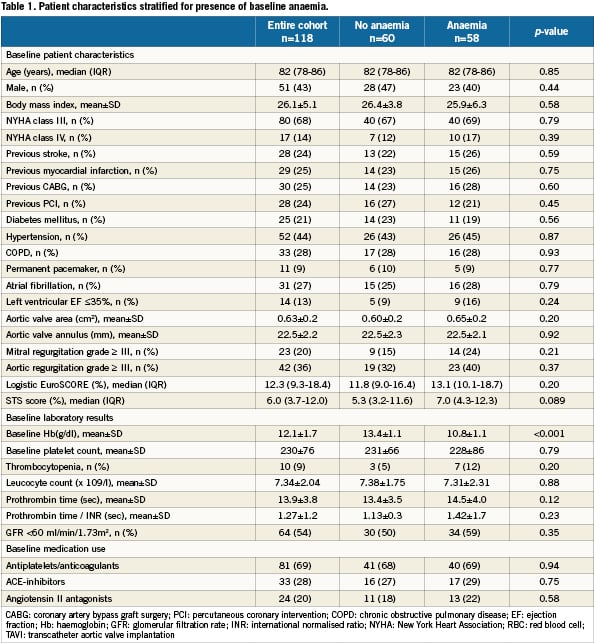
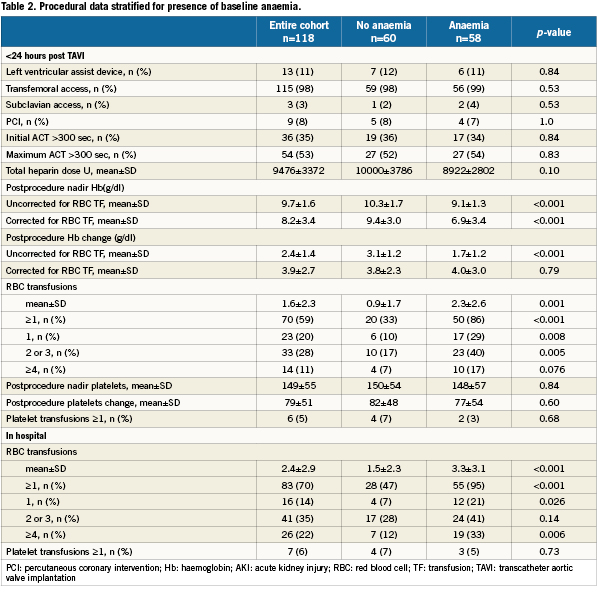
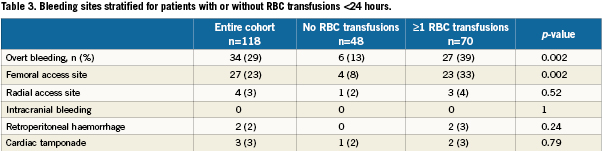
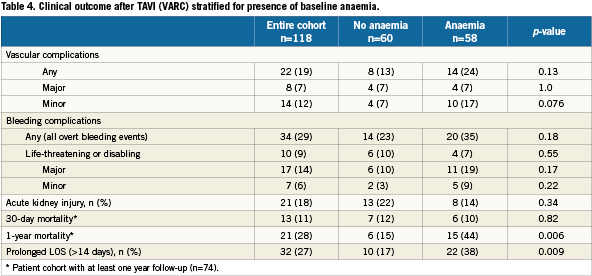
Follow-up
Clinical follow-up was complete for all 118 patients and ranged from one to 52 months, with a median (IQR) of 12 (5-22) months (Table 3). Of the entire cohort 74 had a follow-up exceeding one year. Thirty-seven patients (31%) died during the follow-up period at a median (IQR) of 171 (30-325) days after the TAVI. Cause of death is depicted in Table 5. For the cohort with at least one year follow up, there was no difference in 30-day mortality but a significantly higher mortality at one year in patients with baseline anaemia in comparison with patients without baseline anaemia (44% versus 15%, p=0.006, Table 4, myelodysplastic). By univariate analysis, death was more prevalent in patients with baseline anaemia and thrombocytopenia, in patients undergoing concomitant TAVI with PCI, in patients with more RBC transfusions and with AKI. By multivariate analysis, baseline anaemia and AKI were identified as independent predictors of cumulative late mortality (Table 6, Figure 1).
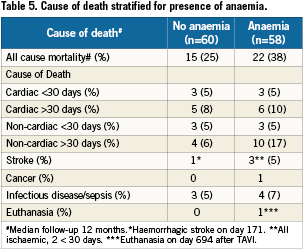
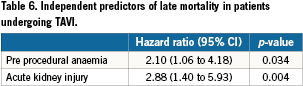
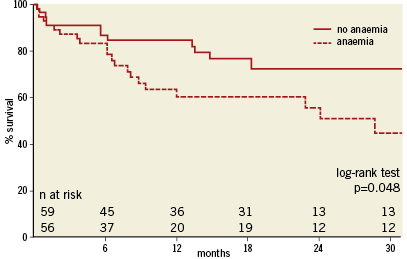
Figure 1. Kaplan-Meier survival curves with or without baseline anaemia.
Discussion
In this single-centre study the prevalence of baseline anaemia in patients undergoing TAVI was 49%. Baseline anaemia was identified as an independent predictor of 1-year mortality. Patients with baseline anaemia received significantly more RBC transfusions although there was no excess in net blood loss or in periprocedural vascular complications.
The herein reported prevalence of baseline anaemia of 49% is higher than reported in patients undergoing coronary artery bypass or valve surgery (16 to 36%) and in patients with an acute coronary syndrome (14 to 43%)11,12,19-22. This is most likely explained by the fact that patients currently selected for TAVI are older and considered too high a risk for surgery due to co-morbidity. A higher rate of anaemia in such patients may, therefore, be anticipated23. The aetiology of anaemia in these patients is multi-factorial and may be caused by nutritional deficiency (iron, vitamin B12, folate), the presence of chronic (e.g., diabetes, renal failure) and inflammatory diseases (e.g., rheumatoid arthritis), any myelodysplastic syndrome in addition to occult bleeding from the gastrointestinal tract23-25. Often the cause of anaemia is unknown. In a large US database the cause of anaemia was unknown in up to one third of anaemic individuals aged 65 years or older23.
Baseline anaemia was found to be an independent predictor of one year mortality
Whether this finding is explained by a causal relationship remains to be established. The prognostic implication of baseline anaemia has been reported in large series of patients with other cardiac diseases and interventions. In an analysis encompassing almost 40,000 patients enrolled in 16 ACS trials, baseline anaemia was found to independently predict 30-day major adverse cardiovascular events9. In a study of 6,025 consecutive patients undergoing PCI because of stable or unstable angina pectoris, baseline haematocrit was found to predict one year mortality8. A relationship between baseline anaemia and both immediate and long-term outcome has been reported in patients undergoing cardiac surgery10-12. Using the same WHO definition of anaemia in a multicentre cohort of 3,500 patients, Karkouti et al demonstrated preoperative anaemia was a strong predictor of in-hospital adverse outcome after cardiac surgery (CABG and/or valve surgery)12. Van Straten et al, who also used the WHO definition of anaemia, reported baseline anaemia were an independent predictor of both early and late mortality11.
We found that patients with baseline anaemia received more RBC transfusions in comparison to patients without baseline anaemia despite a similar drop in the corrected haemoglobin level (as a surrogate for net blood loss) and frequency of vascular and bleeding complications. Expectedly the resultant Hb level will fall lower in case of baseline anaemia and there may be a potential lower threshold and bias to administer RBC transfusion(s) in the absence of a strict protocol. The cause of bleeding during or after TAVI may be multi-factorial and bleeding may be overt, occult (retroperitoneal) or remote (e.g., intracranial, intrapericardial). An arbitrary haemoglobin cut-off to trigger RBC transfusions should be avoided. Furthermore, RBC are often transfused while vascular or bleeding complications are evolving or during haemodynamic instability without knowledge of actual haemoglobin levels. Packed RBCs contain a variety of bioactive humoral mediators that can have immunosuppressive and pro-inflammatory effects (immunomodulation)26,27. Also storage of RBC induces conformational and biochemical changes in the erythrocytes that may generate increased aggregability and a prothrombotic milieu26. RBC transfusions have already been linked to the occurrence of acute kidney injury after TAVI and multi-organ failure in trauma patients28-31. Longer term effects of RBC transfusion remain speculative. Reserving RBC transfusion for patients with active bleeding and/or low Hb level with concomitant evidence of ischemia or clinical instability seems reasonable. This is supported by data from the ICU literature suggesting that a more restrictive transfusion policy (Hb <7g/dL cutoff) might save lives even in patients with cardiovascular morbidity32,33. The data of the CRUSADE registry and a single-centre cohort of 5,538 consecutive patients who underwent PCI disclose there is no need for RBC transfusions when the nadir haematocrit is 24 to 25%34,35. In elderly patients with acute myocardial infarction, however, RBC transfusion for haematocrit levels <30% seemed beneficial22.
Efforts to correct anaemia in anticipation of TAVI by iron or Erythropoietin (EPO) administration might be tantalising but are fraught with limitations and risks36,37. Especially the thrombotic risk associated with EPO therapy poses a concern.
The main reason for RBC transfusion in our TAVI patient cohort was related to vascular access site complications. Avoiding vascular access site complications is thus essential. Meticulous arterial access technique using echo or fluoroscopy guidance and further device iterations towards smaller sized systems will help limit the vascular complication rate. Whether subclavian rather than transfemoral access would come with less bleeding complications and RBC transfusions cannot be concluded from our data given the limited number of subclavian procedures included but warrants further research. Finally, the recommendation for an antiplatelet regimen containing aspirin and clopidogrel started before the procedure and continued for at least six months is not scientifically based and warrants further research.
Study limitations and clinical implications
The present study is based on a post hoc analysis of prospectively gathered data from a single-centre experience with consequently a relatively small sample size. Therefore, our findings are of an exploratory nature. This is in particular the case for the determination of independent predictors of mortality during the follow-up period. Outcome of cardiac patients who undergo an intervention is complex and multi-factorial. Several clinical and procedural factors may have remained unrecognised in this analysis. The decision to give RBC transfusions was left at the discretion of the treating physician in the absence of a standardised protocol, which may cause inherent bias to our data.
The findings of this study in relation to the prognostic implications of baseline anaemia are in accordance with the existing PCI and cardiac surgery literature. It emphasises the need for dedicated care and prevention of blood loss and vascular complications in patients treated with TAVI.
Conclusions
Baseline anaemia was seen in approximately 50% of our patients selected for TAVI. It is associated with a higher frequency of RBC transfusion and was found to be an independent predictor of 1-year mortality after TAVI.
Conflict of interest statement
The authors have no conflict of interest to declare.

