Abstract
Aims: Transcatheter aortic valve implantation (TAVI) can be associated with varying degrees of new renal impairment. The aim of this multicentre analysis was to determine the impact and predictors of periprocedural acute kidney injury (AKI) on prognosis after TAVI.
Methods and results: From the ClinicalService® (a nation-based data repository and medical care project) dataset, 1,157 patients with severe aortic stenosis treated with the third-generation CoreValve prosthesis in seven Italian sites, and with creatinine data available at baseline and during the post-TAVI in-hospital course, were included in this analysis. All outcomes were defined according to the VARC criteria. Overall, AKI occurred in 231 (20.0%): 15.4% stage 1, 2.7% stage 2, and 1.9% stage 3. Compared to patients without AKI, patients who suffered post-procedural AKI had significantly higher three-year all-cause mortality (31% vs. 12%; adjusted HR: 2.09; 95% CI: 1.52-2.87, p<0.001) and cardiovascular mortality (14% vs. 6%; adjusted HR: 2.28; 95% CI: 1.41-3.71, p=0.001). No significant differences in terms of stroke, spontaneous MI, and bleeding were reported. Female gender (adjusted OR: 1.37, 95% CI: 1.01-1.87; p=0.045), baseline renal insufficiency (adjusted OR: 11.02, 95% CI: 5.12-23.73; p<0.001), general anaesthesia (adjusted OR: 1.37, 95% CI: 1.00-1.87; p=0.050), and transfusion ≥3 red blood cell (RBC) units within 72 hrs from TAVI (adjusted OR: 1.65, 95% CI: 1.02-2.68; p=0.041) were found to be independent predictors of AKI.
Conclusions: Acute kidney injury is a frequent complication and significantly impacts on both early and long-term TAVI survival. Females, subjects with impaired renal function at baseline, patients undergoing TAVI under general anaesthesia, and patients receiving ≥3 RBC units after the procedure should be considered populations at high risk for the development of AKI after TAVI.
Abbreviations
AKI: acute kidney injury
CI: confidence interval
HR: hazard ratio
MI: myocardial infarction
OR: odds ratio
RBC: red blood cells
TAVI: transcatheter aortic valve implantation
VARC: Valve Academic Research Consortium
Introduction
Acute kidney injury (AKI) has been reported in approximately 30% of all patients undergoing cardiac surgery, and it has been considered to be a risk factor and predictor of mortality and morbidity1-4. Though less invasive, transcatheter aortic valve implantation (TAVI) is also associated with varying degrees of post-procedural AKI, having been reported in 12% to 57% of patients undergoing this procedure5-10. Patients undergoing TAVI frequently have abnormal baseline renal function and are, therefore, at increased risk for AKI because of the haemodynamic changes during the procedure and the use of contrast agents. Similarly to the surgical approach, this complication was found to be associated with a poorer prognosis5-10. The aim of this large multicentre analysis was to determine the influence and predictors of periprocedural AKI on prognosis after TAVI using the self-expanding CoreValve prosthesis (Medtronic Inc., Minneapolis, MN, USA).
Methods
STUDY DESIGN AND PATIENT POPULATION
Starting from June 2007, all consecutive patients with severe aortic stenosis (AS) undergoing TAVI with the third-generation 18 Fr CoreValve device (Medtronic Inc.) in seven Italian centres were prospectively included in the ClinicalService® Project [http://clinicaltrials.gov/ct2/show/NCT01007474]. This is a nation-based clinical data repository and medical care project aimed at describing and improving the use of implantable devices in Italian clinical practice. The project was approved by each site’s Institutional Review Board or Medical Director and conforms to the principles outlined in the Declaration of Helsinki. Each patient signed an informed consent for data collection and analysis. Clinical and echocardiographic follow-up were performed at 30 days, one year and then yearly with visits or telephone contacts according to each centre’s clinical practice. All events were site reported. For the purpose of the current analysis, 1,157 consecutive patients who underwent CoreValve implantation, and with creatinine data available at baseline and during the post-TAVI in-hospital course, were included in this analysis (n=88 and n=121 patients were excluded because of the absence of baseline and post-TAVI creatinine data, respectively). Patients on chronic haemodialysis (n=19) were also excluded from this analysis. Eligibility for TAVI was established at each centre based on the consensus of a local multidisciplinary team, including clinical cardiologists, cardiac surgeons, and cardiac anaesthesiologists. All the procedures were approved for compassionate use in patients considered at high risk for surgery. All patients were dichotomised according to development of AKI after the procedure (AKI group vs. no-AKI group).
PROCEDURE
Design features of the CoreValve prosthesis and technical details of the procedures have been previously described11-13. The CoreValve prosthesis, available in 26 mm and 29 mm sizes and, starting from September 2011 and August 2012 respectively, even in 31 mm and 23 mm sizes, was implanted using the transfemoral, subclavian and transaortic approaches with an 18 Fr delivery catheter, later improved by an AccuTrak Stability Layer (Medtronic Inc.). All procedures were performed under local anaesthesia (with or without additional sedation and/or analgesia) or general anaesthesia and endotracheal intubation, under fluoroscopic guidance, in a standard cardiac catheterisation laboratory with surgical back-up or in a hybrid operating room. In patients with baseline renal insufficiency, AKI prevention protocols were carried out according to the local institutional policies.
DEFINITIONS
Acute kidney injury was defined according to the Valve Academic Research Consortium (VARC) recommendations as an absolute (≤72 hours) reduction in kidney function and defined as: stage 1 - increase in serum creatinine to 150-200% (1.5-2.0 x increase compared with baseline) OR increase of ≥0.3 mg/dL (≥26.4 mmol/L); stage 2 - increase in serum creatinine to 200-300% (2.0-3.0 x increase compared with baseline); stage 3 - increase in serum creatinine to ≥300% (>3 x increase compared with baseline) or serum creatinine of ≥4.0 mg/dL (≥354 mmol/L) with an acute increase of at least 0.5 mg/dL (44 mmol/L) OR the new need for renal replacement therapy post TAVI14. Cardiovascular mortality, stroke, myocardial infarction (MI) and bleeding were also reported according to VARC criteria14.
STATISTICAL ANALYSIS
Descriptive statistics are reported as mean±1 standard deviation for normally distributed continuous variables, as median and 25th-75th percentile (IQR) otherwise. Normality of distribution was tested by means of the Kolmogorov-Smirnov test. Absolute and relative frequencies are reported for categorical variables. Continuous Gaussian variables were compared by means of Student’s t-test for independent samples, while skewed distributions were compared using the Mann-Whitney non-parametric test. Differences in proportions were compared by applying chi-square analysis. Differences in 30-day outcomes were assessed using logistic regression.
Logistic models were tested in order to detect predictors of AKI. Results were reported in terms of odds ratio (OR) with 95% confidence intervals (95% CI). Variables with probability value <0.10 in the univariate analysis together with main demographic characteristics were used to determine a set of independent predictors of AKI.
Time to long-term outcomes was described by means of the Kaplan-Meier curve and compared between groups by means of the log-rank test. In case of comparisons among three groups, the Bonferroni method was applied to correct the p-value.
Cox models were fitted considering all variables from Table 1 and Table 3, and hazard ratios (HR) with 95% CI were computed. The proportional hazard assumptions were tested by means of Schoenfeld residuals. A Cox multivariate analysis including all variables with probability value <0.20 in each Cox univariate analysis (AKI, gender, chronic renal insufficiency, prior stroke, atrial fibrillation, prior myocardial infarction), together with important procedural characteristics (general anaesthesia, second CoreValve deployment), was used to determine independent predictors of the outcomes.
Landmark analyses15 were also performed with the pre-specified windows of date of the procedure to day 30 and from day 30 up to three years. Freedom from the outcomes was assessed by means of the Cox proportional hazards model for both 30-day and three-year landmark points.
All data were processed using the Stata 12.1 (Stata Corporation, College Station, TX, USA). The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agreed to the manuscript and vouch for the completeness and accuracy of the data gathering and analysis.
Results
Among 1,157 patients, post-procedural AKI occurred in 231 (20%): 15.4% stage 1, 2.7% stage 2 and 1.9% stage 3. The clinical and echocardiographic characteristics of the population are summarised in Table 1. Mean age was 82±7 years and all patients had severe symptomatic aortic stenosis (mean transaortic gradient 51±15 mmHg, mean aortic valve area 0.40 cm2 [IQR 0.30-0.50]). Compared with patients not experiencing post-procedural renal impairment, patients with AKI were more frequently females (60% vs. 52%, p=0.020) and with baseline chronic renal failure (97% vs. 74%, p<0.001). There were no differences between these two groups in other preoperative variables.
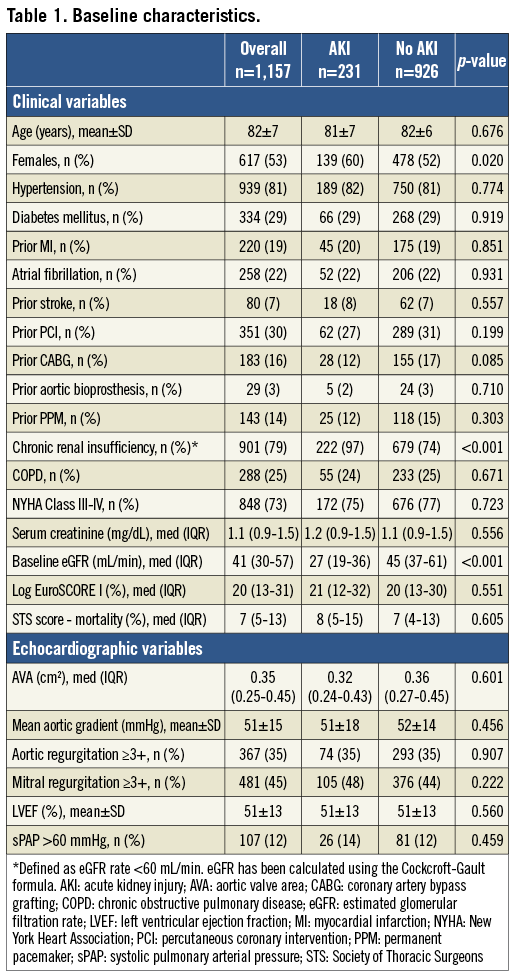
PREDICTORS OF AKI
At multivariate analysis, female gender (adjusted OR: 1.37, 95% CI: 1.01-1.87; p=0.045), chronic renal failure (adjusted OR: 11.02, 95% CI: 5.12-23.73; p<0.001), general anaesthesia (adjusted OR: 1.37, 95% CI: 1.00-1.87; p=0.050), and transfusion ≥3 red blood cell (RBC) units (adjusted OR: 1.65, 95% CI: 1.02-2.68; p=0.041) were found to be independent predictors of AKI. A trend towards a higher risk of AKI was also noted in patients receiving a second prosthesis during the procedure (adjusted OR: 1.88, 95% CI: 0.97-3.42; p=0.061) (Table 2). Figure 1 shows baseline eGFR and creatinine value distribution in patients without AKI and AKI grade 1, 2 and 3.
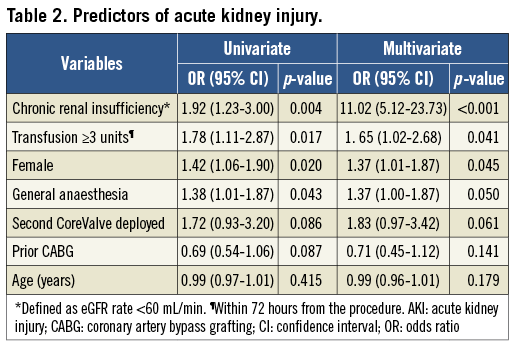
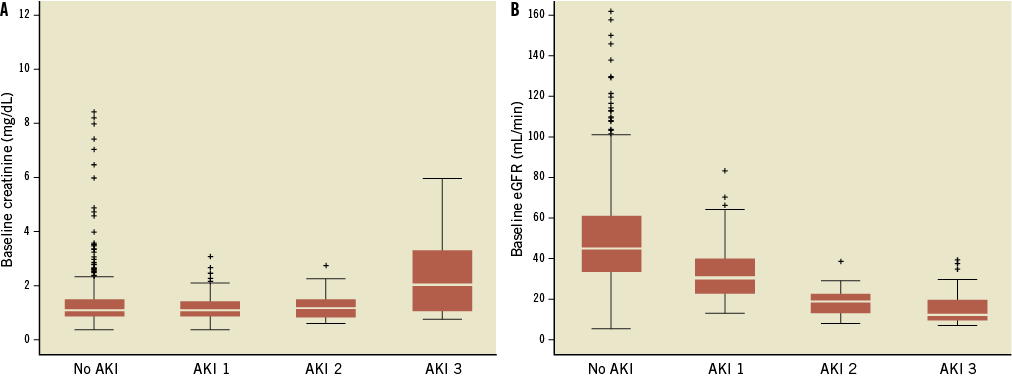
Figure 1. Distribution of baseline creatinine (A) and eGFR (B) values in patients without AKI and those who had TAVI complicated with AKI 1, 2 and 3.
PROCEDURAL AND 30-DAY OUTCOMES
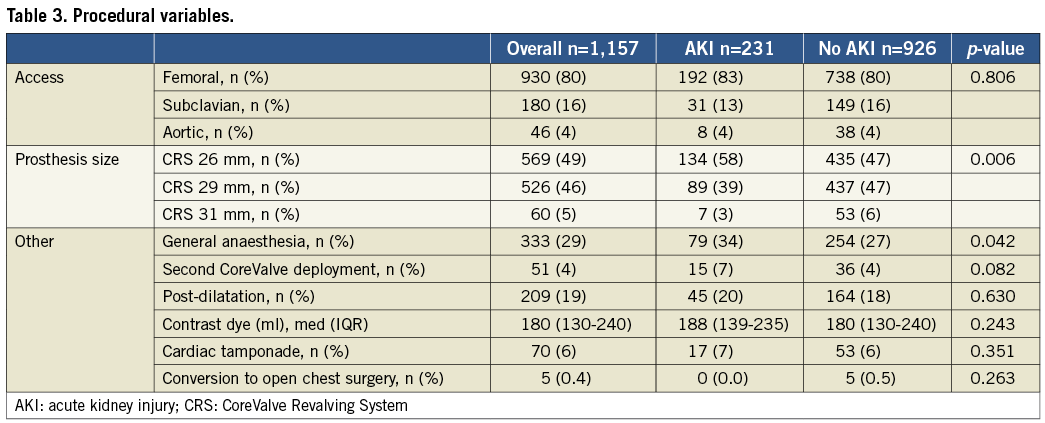
The main procedural variables are presented in Table 3. The transfemoral approach was the most frequently used (80%), followed by the trans-subclavian (16%) and transaortic (4%), with no differences between the two groups (p=0.806). No significant differences in terms of contrast dye administered were reported (median: 188 [IQR 139-235] vs. 180 [IQR: 130-240], p=0.243). Patients who had AKI more frequently underwent TAVI under general anaesthesia (34.2% vs. 27.4%, p=0.042) and received transfusions of ≥3 red blood cell units (11.7% vs. 6.9%, p=0.016) within 72 hours. AKI patients also reported a trend towards a higher rate of second valve implantation (6.5% vs. 3.9%, p=0.082). At 30 days, patients who suffered AKI had a higher all-cause mortality (10.0% vs. 2.6%, p<0.001), cardiovascular mortality (6.5% vs. 1.4%, p<0.001), MI (2.6% vs. 0.9%, p=0.040), and VARC-defined life-threatening bleeding (10.4% vs. 5.8%, p=0.015). Otherwise, there were no differences between groups in terms of cerebrovascular events (2.2% vs. 1.9%, p=0.830) (Table 4).
THREE-YEAR OUTCOMES
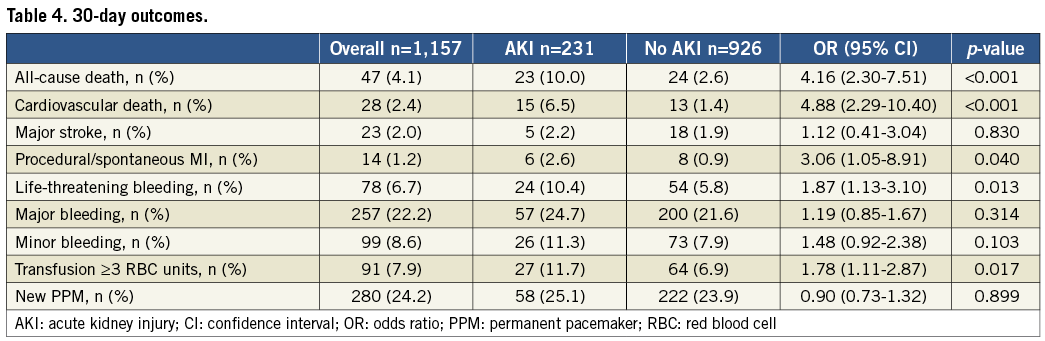
Clinical follow-up was available in 78.3% of patients at a median of 12 months (25th-75th: 2-24 months) after TAVI. Among the AKI group, all-cause mortality was reported in 28%, 32% and 55% of patients, in AKI stage 1, 2 and 3, respectively (p<0.001); cardiovascular mortality was reported in 13%, 13% and 23% of patients, in AKI stage 1, 2 and 3, respectively (p<0.001). Compared to patients without AKI, patients who suffered post-procedural AKI had a significantly higher three-year all-cause mortality (31% vs. 12%; adjusted HR: 2.09; 95% CI: 1.52-2.87, p<0.001) and cardiovascular mortality (14% vs. 6%; adjusted HR: 2.28; 95% CI: 1.41-3.71, p=0.001) (Figure 2). No significant differences in terms of a composite of all VARC-defined bleeding (36% vs. 28%, adjusted HR: 1.24; 95% CI: 0.97-1.60, p=0.087), stroke (3.0% vs. 3.5%, adjusted HR: 0.79; 95% CI: 0.34-1.79, p=0.566), and spontaneous MI (3.0% vs. 1.7%, HR: 1.51; 95% CI: 0.60-3.81, p=0.379) were reported (Figure 3).
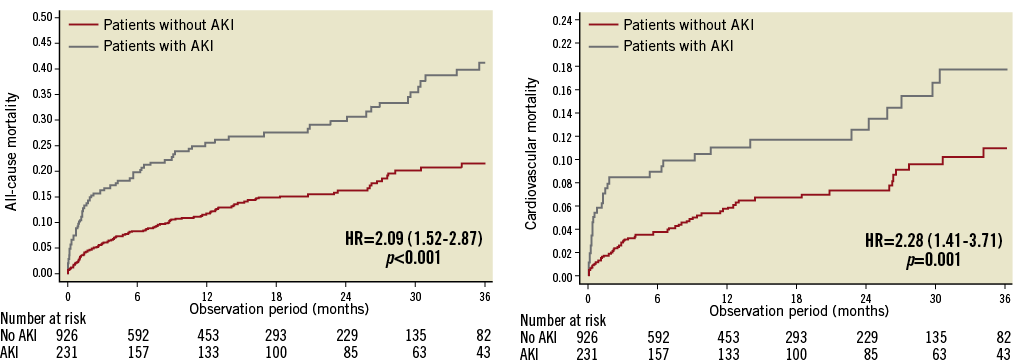
Figure 2. Kaplan-Meier curves showing cumulative and cardiovascular mortality rates through three years. Comparison of the cumulative (A) and cardiovascular (B) death rates through three years in patients with AKI compared with patients without AKI. Adjusted HRs (95% CI) are reported.
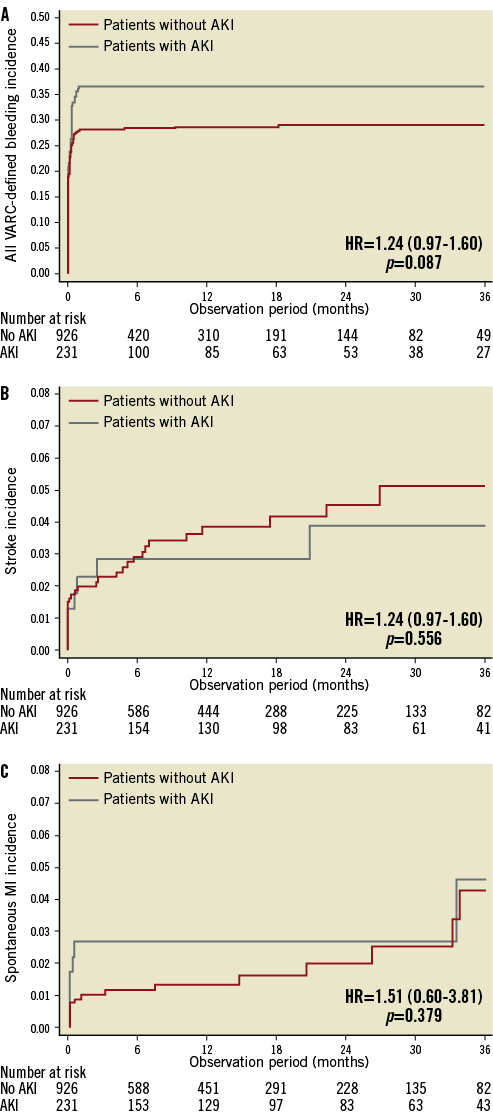
Figure 3. Kaplan-Meier curves showing stroke, myocardial infarction and all bleeding rates through three years. Comparison of all bleeding (A), myocardial infarction (B) and the major and minor stroke (C) rates through three years in patients with AKI compared with patients without AKI. Adjusted HRs (95% CI) are reported.
LANDMARK ANALYSIS
The 30-day landmark analyses for all-cause and cardiovascular mortality are shown in Figure 4. A consistent pattern of significant all-cause mortality difference in the AKI vs. no-AKI group was found both during the first 30 days (HR: 3.85; 95% CI: 2.16-6.82, p<0.001), and from 30 days up to three years (HR: 1.85; 95% CI: 1.30-2.63, p=0.001). By contrast, while a significantly higher 30-day cardiovascular mortality was reported in patients with AKI (HR: 4.96; 95% CI: 2.21-9.75, p<0.001), no significant difference was found between 30 days and three years (HR: 1.32; 95% CI: 0.76-2.27, p=0.327).
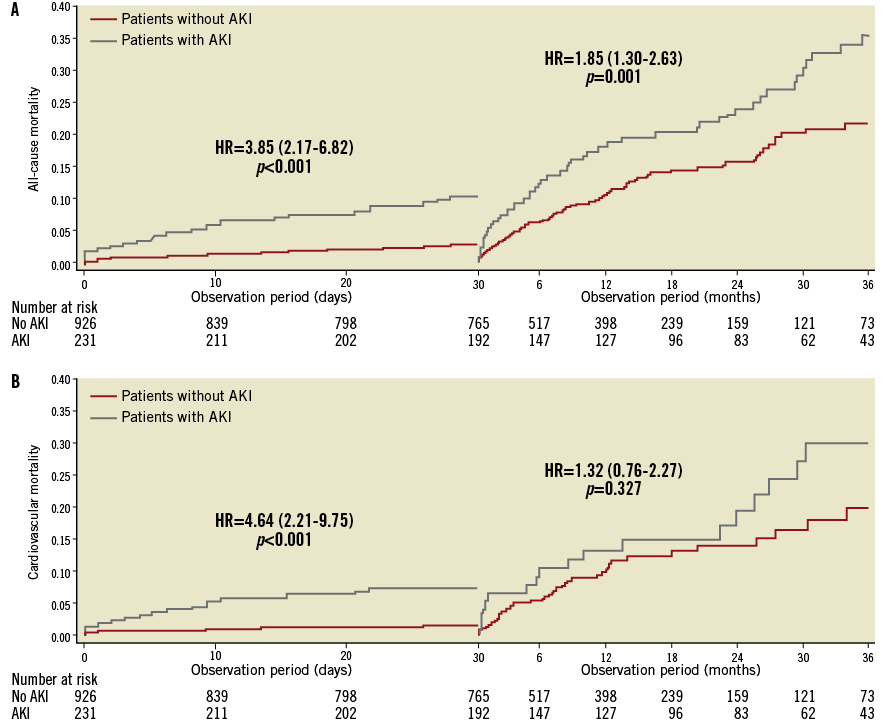
Figure 4. Landmark analyses of all-cause and cardiovascular mortality. Landmark analyses (survival method) of the Kaplan-Meier estimates of all-cause (A) and cardiovascular (B) mortality during the first 30 days after the procedure (left side of each graph) and from 30 days to 3 years (right side of each graph) are shown for patients with post-procedural AKI and without.
Discussion
Renal function should be kept in mind in elderly and sick patients such as those undergoing TAVI, since post-procedural AKI is associated with increased short-term and long-term mortality and morbidity5-10. This large multicentre analysis adds considerably to the current knowledge of kidney function after TAVI using the CoreValve device with the following observations: i) AKI complicated one fifth of TAVI procedures; ii) this complication significantly impacted on both early and long-term TAVI survival; iii) females, subjects with impaired renal function at baseline, patients undergoing TAVI under general anaesthesia, and patients receiving ≥3 RBC units after the procedure should be considered populations at high risk for the development of AKI after TAVI.
The incidence of AKI, as defined according to VARC criteria, was not infrequent after TAVI occurring in 20% of the entire cohort. This percentage is consistent with a recent meta-analysis which reported a pooled estimated rate of VARC-defined AKI after TAVI of 20.4%16. Similarly, Nuis et al reported on an AKI incidence of 21% among 995 patients with aortic stenosis undergoing TAVI8. The data of these three large analyses suggest that the rate of 20-21% most likely reflects the real incidence of AKI encountered in current clinical practice after TAVI. Fortunately, severe worsening of renal function (AKI stage 2 and 3) was infrequent, occurring in 2% and 3% of cases, respectively. These results also echo those reported in the literature8-10,16. The VARC report recommended evaluating and classifying AKI ≤72 hours after TAVI14. However, it should be pointed out that there is evidence showing that the peak creatinine value after TAVI is often reached after 72 hours. This means that a proportion of AKI might have been misdiagnosed or underclassified. As a consequence, in the new VARC-2 criteria, the timing for the diagnosis of AKI has been extended from 72 hours to seven days after the procedure17.
Secondly, acute kidney injury, particularly stages 2 and 3, significantly impacted on long-term outcomes after TAVI. Compared to patients without AKI, patients who suffered post-procedural AKI had more than a twofold increased risk of all-cause (31% vs. 12%) and cardiovascular (14% vs. 6%) mortality. In addition, the exploratory landmark analyses reported here showed that there is a consistent significant increased risk of all-cause mortality both early (<30 days) and late (≥30 days) after the procedure. The effect of post-procedural AKI mostly emerged early (almost fourfold), and continued to accrue during long-term follow-up, though with a lower effect (almost twofold). These results differ slightly from those reported by Généreux and co-workers who showed an impact of VARC-defined AKI only at 30 days10; however, the findings of this latter study were limited by the small number of patients included. On the other hand, the impact of AKI on cardiovascular mortality was evident only at 30 days (almost fivefold increased risk), while it was not between 30 days and three years.
This analysis also evidenced four important predictors of post-procedural AKI: female gender, baseline chronic renal failure, general anaesthesia and transfusions of ≥3 RBC units. The association between blood transfusions and AKI has been extensively investigated in either surgical or percutaneous aortic valve replacement series5,8,18. More recently, Nuis et al added to this evidence with the finding that the higher the number of RBCs transfused, the more elevated the odds of AKI, suggesting that TAVI outcome may be improved by a more careful use of blood transfusions8. Similarly, it has been widely demonstrated that patients with impaired renal function at baseline are at high risk for AKI1,2,19,20. On the other hand, the relationship between female gender, general anaesthesia and AKI in the context of TAVI has never been documented. Female sex has been demonstrated to be associated with increased odds of AKI after cardiac procedures21. The relationship between AKI and general anaesthesia may be explained by the effects of temporary hypoperfusion and systemic inflammatory response22,23. Therefore, these findings suggest that local anaesthesia and sedation may minimise intraoperative renal insults and maintain isovolaemia, adequate cardiac output, and renal perfusion pressure, thus reducing the risk of post-operative AKI. Interestingly, despite a large body of evidence to correlate the amount of contrast dye and risk for contrast-induced nephropathy24, this correlation was not found in our analysis. These results tend to support the hypothesis that the pathophysiology of AKI after TAVI is more likely multifactorial, involving predisposition of some patients to injury, contrast nephropathy, embolic phenomena and injury by hypoperfusion. Nonetheless, continued efforts to minimise the amount of contrast media in these patients are strongly recommended to reduce the risk of AKI following TAVI.
Limitations
The present analysis has several main limitations: firstly, AKI was defined according to VARC-1 criteria which consider an outer bound of 72 hours from the index procedure for diagnosing AKI. However, there is evidence showing that the peak creatinine value after TAVI is often reached after 72 hours. This means that in the present analysis a proportion of AKI might have been misdiagnosed or underclassified. Secondly, the pathophysiologic mechanisms of AKI after TAVI cannot be derived from this analysis; in addition, data on haemodynamic instability which occurred during the procedure were not available. Thirdly, the heterogeneity of AKI prevention protocols across centres might have affected the real incidence of AKI; however, an analysis of AKI incidence broken down by all the centres revealed no significant differences. Finally, the absence of an independent monitoring and external adjudication of the events might limit the strength of this analysis.
Conclusions
Acute kidney injury is a frequent complication and significantly impacts on both early and long-term TAVI survival. Females, subjects with impaired renal function at baseline, patients undergoing TAVI under general anaesthesia and patients receiving ≥3 RBC units after the procedure should be considered populations at high risk for the development of AKI after TAVI.
Acknowledgements
The authors would like to thank Alessio Marseglia (Medtronic Inc.) for his important contribution in data extraction and technical support.
Conflict of interest statement
Medtronic Italy is the sponsor of the ClinicalService® Project. L. Mangoni and A. Rossi are employees of Medtronic Italia, an affiliate of Medtronic Inc. A. Latib, F. Maisano, F. Bedogni, F. Ettori, A.S. Petronio and G.P. Ussia are consultants for Medtronic Inc. All other authors have no conflicts of interest to declare.

