Abstract
Aims: The aim of this study was to assess the influence of chronic kidney disease (CKD) classification on clinical outcomes in patients undergoing transcatheter aortic valve implantation (TAVI).
Methods and results: Data of 2,929 consecutive patients undergoing TAVI in the FRANCE 2 registry were analysed. Patients were divided into five groups: CKD 1+2, 3a, 3b, 4, and 5. Both 30-day and one-year mortality rates were significantly increased and positively correlated with CKD severity in all groups. After adjusting for significant influential confounders in a Cox regression multivariate model, CKD 4 and 5 were associated with increased risk of both 30-day mortality and one-year mortality when compared with CKD 1+2 as the reference. This higher mortality was predominantly driven by renal failure and infection in patients with CKD 4 and 5, respectively. Procedural success rate in CKD 5 was significantly lower than that in other groups. All CKD patients trended towards a higher incidence of acute kidney injury (AKI), in parallel with the degree of CKD severity.
Conclusions: Classification of CKD stages before TAVI allows risk stratification for 30-day and one-year clinical outcomes. CKD 3b, 4 and 5 correlate with poor outcome and are considered a significant risk for TAVI.
Introduction
Transcatheter aortic valve implantation (TAVI) is a relatively new treatment for severe, symptomatic aortic stenosis and is advocated as a less invasive alternative to conventional surgical aortic valve replacement (SAVR) in patients who do not qualify for surgery1-3. Aortic stenosis is a well-recognised complication of renal dysfunction4. With a prevalence of 13% in the developed world, chronic kidney disease (CKD) is a significant factor increasing the risk of cardiovascular complications, specifically aortic stenosis5. Current guidelines provide a classification of CKD stages based on estimated glomerular filtration rate (eGFR): stage 1, >90 ml/(min·1.73 m2); stage 2, 60-90 ml/(min·1.73 m2); stage 3a, 45-60 ml/(min·1.73 m2); stage 3b, 30-45 ml/(min·1.73 m2); stage 4, 15-30 ml/(min·1.73 m2); and stage 5, <15 ml/(min·1.73 m2)6. CKD has a considerable effect on the presence and severity of aortic stenosis7. Several small-scale studies have examined the outcome of TAVI in CKD patients8-10. They reported that CKD was not associated with a higher risk of mortality in TAVI, despite a high risk of cardiovascular disease in CKD patients. A previous study evaluating 642 patients who underwent TAVI indicated that patients with CKD stage 4 were associated with the greatest risk of mortality11. However, the subgroup with CKD stage 5 had been excluded in the former analysis, as well as from most of the studies to date, including the PARTNER I and II, CoreValve US Pivotal, and SURTAVI trials. Recent studies conducted by the French Aortic National CoreValve and Edwards 2 (FRANCE 2) registry have included large numbers of patients12. The current study is a sub-analysis of the FRANCE 2 registry, and compares the clinical outcomes among the CKD stages, including stage 5, after TAVI was performed.
Methods
PATIENT SELECTION
In January 2010, a national TAVI coordination and monitoring programme was established in France to analyse patient characteristics and clinical outcomes in 33 medical centres in France and one centre in Monaco12. Eligibility for TAVI was based on systematic clinical, angiographic, multislice computed tomographic and echocardiographic assessments. The eGFR value was calculated using the Modification of Diet in Renal Disease (MDRD) equation, where eGFR (expressed in ml/[min·1.73 m2])=186×(serum creatinine)1.154×(age)0.203×(0.742 if the patient was female)13. Based on these clinical criteria, TAVI was performed in 3,195 patients in 34 hospitals between January 2010 and October 2011. Of these patients, 266 were excluded from the initial analysis because of missing data for both serum creatinine and dialysis. For prospective comparison, data from the remaining 2,929 patients were classified into five groups on the basis of the eGFR as follows: CKD 1+2, >60 ml/(min·1.73 m2); CKD 3a, 45-60 ml/(min·1.73 m2); CKD 3b, 30-45 ml/(min·1.73 m2); CKD 4, 15-30 ml/(min·1.73 m2); and CKD 5, <15 ml/(min·1.73 m2). Eighty-two patients (2.8% of total population) receiving regular haemodialysis (HD) before TAVI were included in the CKD 5 group.
TAVI
The technical aspects of the TAVI procedure have been previously reported in detail2,3. In brief, two TAVI systems are commercially available: a self-expandable prosthesis (the Medtronic CoreValve® ReValving System; Medtronic, Minneapolis, MN, USA) and a balloon-expandable prosthesis (the Edwards SAPIEN valve; Edwards Lifesciences, Irvine, CA, USA). No pre-specified recommendations were made regarding the use of a transfemoral, transapical, or subclavian approach.
DATA MANAGEMENT
All adverse events were assessed according to the Valve Academic Research Consortium (VARC) classification14. VARC criteria were used to evaluate device success and 30-day combined safety. The VARC classifications were also used to assess other procedural complications during TAVI. Acute kidney injury (AKI) stage 2 was defined as an increase of 200-300% (2.0-3.0×increase compared with baseline) in serum creatinine or as an increase of ≥0.3 mg/dL (≥26.4 mmol/L) and ≤4.0 mg/dL (≤354 mmol/L) compared with baseline (up to 72 hrs). AKI stage 3 was defined as changes exceeding those observed in stage 2. Post-procedural aortic and mitral regurgitation were assessed by echocardiography.
Mortality was evaluated by an independent clinical events committee. Cause of death was classified according to the VARC classification14, which specifically denotes cardiovascular mortality as an important secondary endpoint and subdivides it according to the following criteria:
– Any death due to proximate cardiac cause (e.g., myocardial infarction [MI], cardiac tamponade, and worsening heart failure).
– Unwitnessed death and death of unknown cause.
– All procedure-related deaths, including those related to a complication of the procedure or treatment for a complication of the procedure.
– Death caused by non-coronary vascular conditions such as cerebrovascular disease, pulmonary embolism, ruptured aortic aneurysm, dissecting aneurysm, or other vascular disease.
Non-cardiovascular-related causes of death included renal failure, infection, respiratory failure, cancer, trauma and others.
Data were recorded on a standardised electronic case report form and sent via internet to a central database (Axonal, Nanterre, France). Data were checked against source documents for 10% of patients in randomly selected centres to ensure database quality.
STATISTICAL ANALYSIS
All statistical analyses were performed using the SPSS software, Version 19 (IBM Corp., Armonk, NY, USA.). Continuous variables were expressed as either mean±SD or median, depending on variable distribution. Categorical data were expressed as a percentage of the total. The five groups were compared using Pearson’s χ2 test for categorical covariates, and one-way ANOVA for continuous covariates. Associations of renal function with the endpoints were assessed using a Cox regression model. For this purpose, renal function was categorised into CKD 3a, CKD 3b, CKD 4, and CKD 5, using CKD 1+2 as the reference. The Kaplan-Meier method was used to estimate cumulative mortality rates in the groups. Mortality rates at the 30-day and one-year time periods were also calculated for each group, and compared using the log-rank test.
Cox regression models were used to determine the independent risks of renal function for the following endpoints in the five groups: cumulative mortality at 30 days and one year, procedural success (Success), life-threatening and major bleeding (Bleeding), major vascular complication (VC), and AKI severity of more than grade 2. Univariable Cox regression was performed to obtain hazard ratios (HR) for 30-day mortality after TAVI for Success, Bleeding, VC, and AKI severity of more than grade 2. Multivariate regression analysis was subsequently performed using the variables whose p-values were <0.10 in univariate analysis (model 1), or baseline patient characteristics with p-values <0.10 (model 2). The baseline patient characteristics variables with p-values <0.10 (model 2) are shown in Table 1 and Table 2. All statistical tests were two-sided, and a p-value <0.05 was considered significant.
Comparisons of mortality among the five groups were performed using Pearson’s bivariate test and the χ2 test, followed by Tukey’s honestly significant difference (HSD) test on the significant variables. The Cox proportional hazards assumption was validated by using the parallel curves in the log-log plot of the five CKD groups.
Results
BASELINE PATIENT CHARACTERISTICS
Demographic data and baseline associations of the 2,929 study patients are summarised in Table 1. Mean age was 82.8±7.2 years, logistic EuroSCORE was 21.9±14.2, and men comprised 51.5% of the study population. More than half of all patients (52.7%) were categorised as having moderate-to-severe impairment of kidney function, defined as an eGFR <60 ml/(min·1.73m2) (CKD 3a or greater). Figure 1 illustrates the percentage distribution of patients in the five groups. CKD 5 patients were younger than those in other groups. Decrease in eGFR was associated with increased incidence of peripheral artery disease, diabetes mellitus, hypertension, and active smoking. Other significant differences across the five study groups included NYHA III and IV, prior cardiac surgery, logistic EuroSCORE, STS score, atrial fibrillation, and permanent pacemaker implantation. The echocardiographic data showed significant intergroup variations in LVEF, mPG, PAP, and the proportion of greater than mild post-procedural MR.
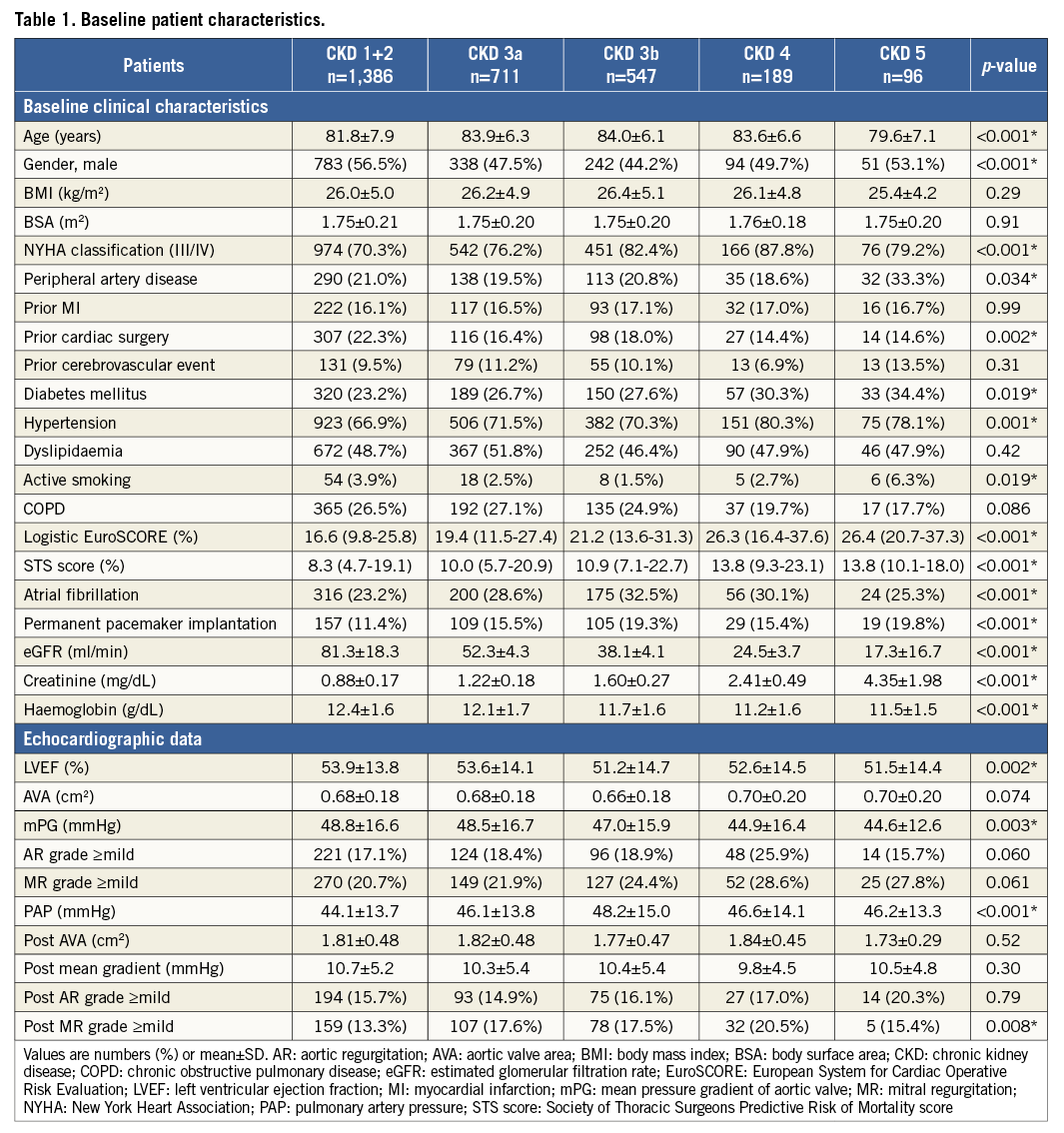
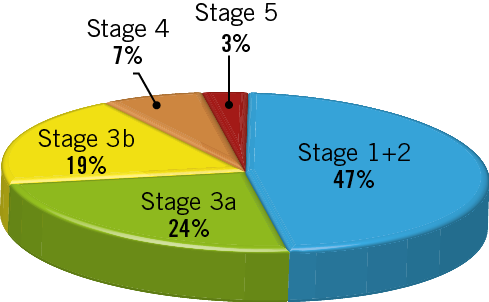
Figure 1. Chronic kidney disease (CKD) classification based on the estimated glomerular filtration rate (eGFR). According to the eGFR value, the individuals were divided into five groups: CKD 1+2, >60 ml/(min·1.73 m2); CKD 3a, 45-60 ml/(min·1.73 m2); CKD 3b, 30-45 ml/(min·1.73 m2); CKD 4, 15-30 ml/(min·1.73 m2); and CKD 5, <15 ml/(min·1.73 m2).
PROCEDURAL CHARACTERISTICS AND OUTCOMES
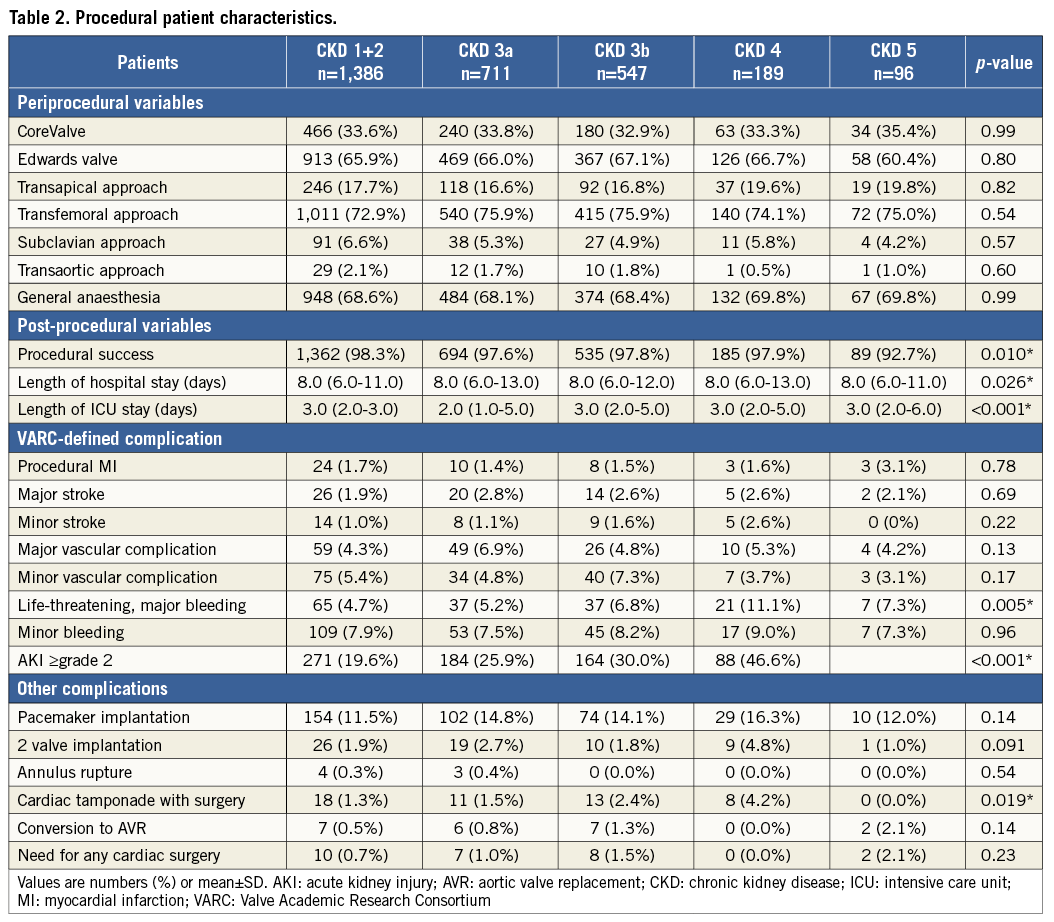
Procedural characteristics are shown in Table 2. The Edwards valve was used in 1,933 patients (66.0%) and the CoreValve in 983 (33.7%). The transapical approach was used in 512 patients (17.5%) and the transfemoral approach in 2,178 (74.4%). Procedural success was significantly lower in CKD 5 (p=0.010). Length of hospital stay was significantly greater in CKD 4, and ICU stay was significantly greater in CKD 5 (p<0.001, p<0.001, respectively). CKD patients showed a significant trend towards a higher incidence of major bleeding and cardiac tamponade (p=0.005 and p<0.001, respectively). The incidence of AKI ≥2 paralleled the degree of CKD severity (p<0.001).
INCIDENCE OF CUMULATIVE MORTALITY
A total of 452 patients (15.4%) died during one year, at a median of 151 days (interquartile range, 33 to 282 days). Death occurred within 30 days in 231 of these patients (51.1%) and after 30 days in 221 patients (48.9%). Kaplan-Meier analysis of cumulative mortality in the five groups based on renal function is presented in Figure 2. Cumulative 30-day and one-year mortality rates in each individual group were 6.4%, 6.8%, 9.4%, 15.9%, and 24.2%, and 19.4%, 18.8%, 25.0%, 42.0%, and 39.9%, respectively. The probability of cumulative mortality over the entire follow-up period after TAVI was similar between CKD 1+2 and CKD 3a (p=0.99). In contrast, the mortality rates of CKD 3b, 4, and 5 were significantly higher in comparison with CKD 1+2 as a reference (p=0.002, <0.001, and <0.001, respectively).
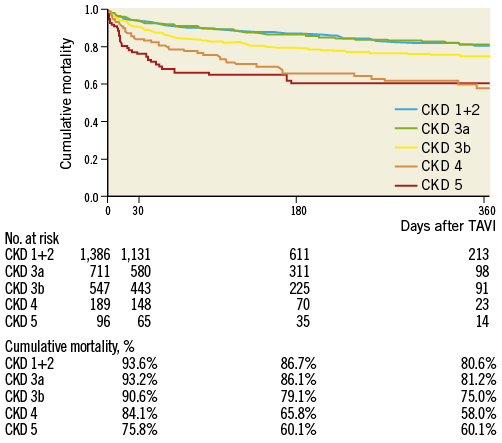
Figure 2. Time-to-event curves for cumulative mortality. The mortality rate was calculated using Kaplan-Meier methods and compared using the log-rank test according to the chronic kidney disease classification.
OVERALL, EARLY, AND LATE MORTALITY
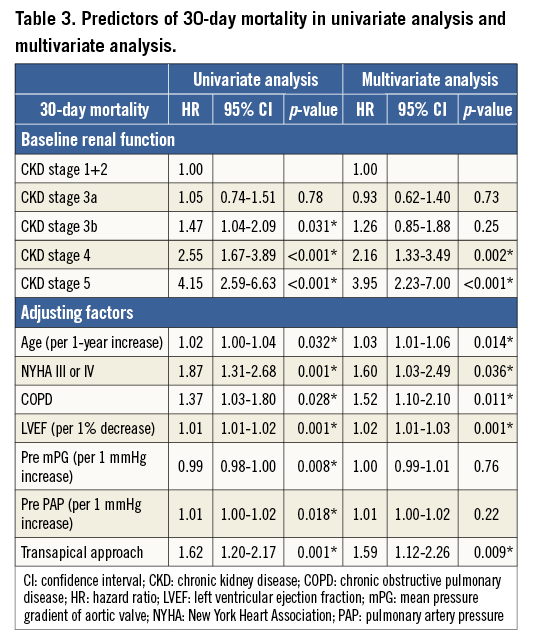
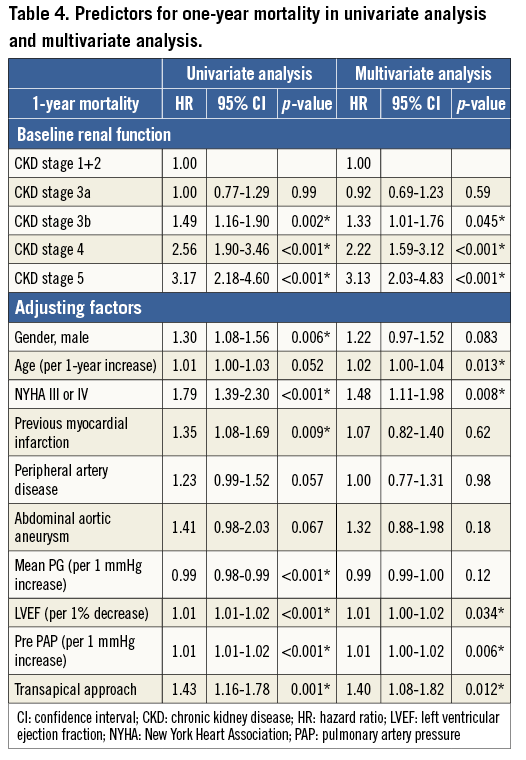
Results of the Cox regression analysis for associations with 30-day and one-year mortality are presented in Table 3 and Table 4, respectively. A Cox multivariate regression analysis was performed using variables with p-values <0.10 in univariate analysis. The following significant factors remained in the final model: CKD 4 (HR: 2.16, p=0.002), CKD 5 (HR: 3.95, p<0.001), age (HR: 1.03, p=0.014), NYHA (III/IV) (HR: 1.60, p=0.036), COPD (HR: 1.52, p=0.011), LVEF (HR: 1.02, p=0.001), and TA approach (HR: 1.59, p=0.009) for 30-day mortality; and CKD 3b (HR: 1.33, p=0.045), CKD 4 (HR: 2.22, p<0.001), CKD 5 (HR: 3.13, p<0.001), NYHA (III/IV) (HR: 1.48, p=0.008), LVEF (HR: 1.01, p=0.034), PAP (HR: 1.01, p=0.006), and TA approach (HR: 1.40, p=0.012) for one-year mortality.
The Cox univariate and multivariate regression analyses in models 1 and 2 for the association between clinical complications and CKD classification are shown in Figure 3. In all models, the success rate was significantly lower in CKD 5 compared with the other groups. Bleeding was significantly increased in CKD 4 alone. No significant difference in major VC related to CKD stage was detected after multivariate regression analysis. All CKD stages showed a trend towards a higher incidence of AKI, correlating directly with the degree of CKD severity.
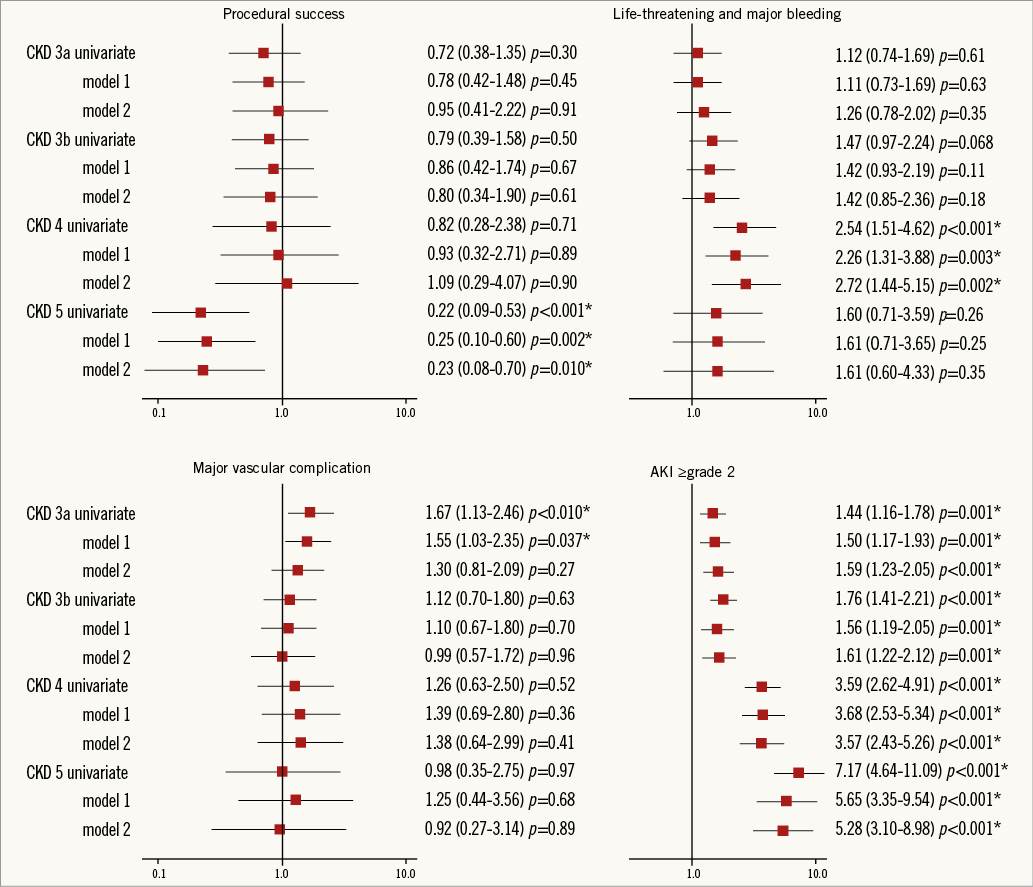
Figure 3. Odds ratio plot (with 95% confidence intervals) for the Valve Academic Research Consortium-defined complications. Cox univariate and multivariate regression analysis of the association between clinical complications and chronic kidney disease classification.
CAUSE OF DEATH
Overall, 461 patients died during the study period, with cardiovascular causes accounting for 288 deaths (63%). There was no significant difference in cardiovascular versus non-cardiovascular mortality (p=0.25 and 0.46, respectively) among the five CKD groups. However, renal failure and infection were significant causes of death in the CKD 4 and CKD 5 groups (p<0.001 and =0.018, respectively) (Figure 4). Incidences of renal failure in CKD 4 and infection in CKD 5 were significantly greater than those in the other CKD groups.
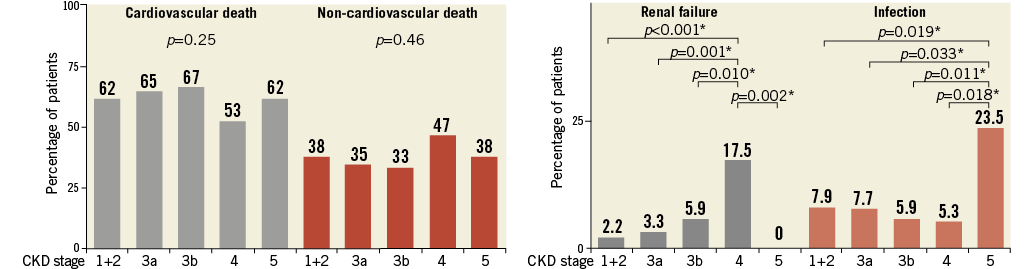
Figure 4. Percentage of all cardiovascular death and non-cardiovascular death among the five chronic kidney disease groups. The causes of mortality were classified according to the Valve Academic Research Consortium classification. The five groups were compared using the Pearson’s χ2 test. In addition, Tukey’s honestly significant difference (HSD) test was performed using the significant variables.
Discussion
The present study demonstrates that CKD classification is a strong predictor of 30-day and one-year clinical outcomes, procedural success, length of hospital stay and AKI in patients undergoing TAVI.
Although the 30-day and one-year outcomes of patients with CKD 1+2 and 3a appeared similar, CKD 3b (eGFR >30 ml/[min·1.73m2]), CKD 4 (eGFR=15-30 ml/[min·1.73m2]) and CKD 5 (eGFR <15 ml/[min·1.73m2]) were associated with an increase in both 30-day and one-year mortality, without attenuation after adjusting for confounding variables. CKD stage 5 was particularly associated with a very high 30-day (24.2%) and one-year (39.9%) mortality rate. These findings indicate that renal failure and infection were significant causes of death in patients with eGFR <30 ml/(min·1.73m2), and suggest that this value may be considered the threshold for predicting 30-day mortality, and eGFR <45 ml/(min·1.73 m2) for one-year mortality.
In the FRANCE 2 registry, increased risk on the logistic EuroSCORE, NYHA functional Class III or IV, the use of a TA approach, and periprosthetic regurgitation grade ≥2 were all reported to be independent predictors of one-year mortality12. The present study showed that CKD 3b, CKD 4 and CKD 5 were additional independent predictors of mortality, a finding that was not attenuated even after adjusting these FRANCE 2 registry predictors in a multivariate model. This result was consistent with that of the previous study, which showed that CKD 3b and 4 were related to increased cumulative one-year mortality in the 642 cases11. Along with CKD 3b, CKD 4 and CKD 5, several other factors in our study were indicated as dependent predictors (age and LVEF for 30-day mortality; LVEF and PAP for one-year mortality). This finding was thought to be different from the original result of the FRANCE 2 registry because we removed the logistic EuroSCORE from the multivariable models after considering multicollinearity.
CKD CLASSIFICATION IN PATIENTS WITH TAVI
CKD classification is a global concept that reflects heterogeneous disorders affecting kidney function. The use of eGFR has recently been espoused as a superior method for evaluating outcomes15. Recent reports on coronary artery bypass graft surgery and SAVR patients have established this method as a reliable predictor of postoperative outcomes16. Several reports have focused on the relationship of CKD staging to clinical outcome in TAVI cohorts8,9. The first single-centre study reported no increase in the 30-day mortality rate of CKD patients (eGFR <60 ml/[min·1.73m2]) compared with non-CKD patients (mortality in the CKD group: 3.9% vs. non-CKD: 8.6%, p=0.3)8. A second multicentre report showed a higher incidence of cardiovascular death in advanced CKD patients (CKD 3 and CKD 4), although this difference was not significant9. The small number of subjects in these studies represents a possible source of error. More precise evaluation of mortality risk might be obtained through stratified analysis by subdividing CKD stage 3 into 3a and 3b, as indicated in recent consensus papers17. The current results suggest that using CKD 3b, CKD 4 and CKD 5 as the optimal cut-off values leads to more accurate prediction of late adverse clinical outcomes.
TAVI IN PATIENTS UNDERGOING HD
This study provides the first precise analysis of prognosis in chronic HD patients with severe AS undergoing TAVI, based on real-world experience in a large number of patients. Calcifications of the aortic valve are present in approximately 28-55% of HD patients, with an average age of onset that is 10 to 20 years lower than that of patients with normal renal function18. HD patients have been excluded from most previous studies because their small numbers resulted in statistical instability. One study evaluated the safety of TAVI in a very small number of HD patients. Although no deaths were reported among 10 dialysis patients and no significant differences in six-month survival rates were noted between HD and non-HD groups, the small population size might have skewed the results10.
In the current study, procedural success was significantly lower and the intensive care unit stay was significantly greater in HD patients. Mortality rates at both 30 days and one year were also significantly greater in HD patients. This might be because HD patients had a high comorbid burden, including a high prevalence of congestive heart failure, stroke, diabetes, peripheral vascular disease, chronic obstructive pulmonary disease, and prior myocardial infarction. HD patients had heavy systemic calcification, in particular severe aortic valve calcification. The extent of aortic valve calcification could influence the success or procedural outcome. Moreover, in HD patients having a hyperdynamic status, the valve may be placed in an inappropriate location.
This study demonstrates that infection was the predominant cause of greater mortality in CKD 5. This finding agrees with that of prior reports showing a greater risk of infection, bacteraemia, sepsis, and mortality among patients receiving long-term HD19. TAVI is still considered a challenging procedure for HD patients. Patients in the CKD 4 group experienced the highest risk for major bleeding, cardiac tamponade with surgery, and the longest duration of hospital stay, beyond that of HD patients. This evidence suggests that CKD 4 was a significant threshold for predicting outcomes, although CKD 5 had the highest mortality.
AKI AFTER TAVI IN CKD PATIENTS
In our study, CKD patients with eGFR <60 ml/(min·1.73 m2) had a significantly greater risk of AKI than did non-CKD patients (p≤0.001). Renal function and the development of AKI are important factors for patient outcome after invasive procedures. This study demonstrates that renal failure was associated with a greater mortality risk in the CKD 4 group. The occurrence of AKI correlated with several causes in addition to baseline renal function20. A decrease in eGFR is not only a marker of illness severity but also an indicator of onset of AKI. AKI itself acts as a causative factor for cardiovascular injury with concomitant activation of neurohormonal, immunological, and inflammatory pathways21. Several strategies for preventing these complex complications have been identified in the literature. For instance, a regimen of pre-procedural and post-procedural hydration therapy with sodium bicarbonate appeared to be more effective than post-procedural hydration therapy alone with isotonic saline22. However, further therapy evaluations and device refinements as well as careful screening are necessary in these high-risk patients.
Study limitations
The following limitations apply to the current study. Although the MDRD equation and the Cockcroft-Gault method are superior to using the serum creatinine level for estimating renal function, their reliability for calculating eGFR value in elderly patients is limited23. The data regarding numbers of procedures and survival outcomes are extremely robust, but those concerning morbidity and complications are likely to be less so, as they are self-reported and not independently substantiated because of the absence of central echocardiography and neurology core laboratories. VARC criteria have been superseded in 2012 by those of VARC-224. However, the data from the FRANCE 2 registry, which had enrolled patients up to mid-2012, were not available to reclassify the patients’ renal status from the RIFLE classification to the AKIN classification. Finally, some differences in clinical backgrounds among the five study groups may not have been accounted for because the FRANCE 2 registry is a non-randomised clinical investigation.
Conclusions
Classification of CKD stages before TAVI allows risk stratification of patients regarding 30-day and one-year clinical outcomes. In the real-world population of the FRANCE 2 registry, CKD 4 and CKD 5 were independent predictors of higher mortality, lower procedural success rate, and prolonged hospitalisation in patients undergoing TAVI. TAVI is therefore considered a challenging procedure for CKD 4 and CKD 5 patients.
| Impact on daily practice The present study analyses the outcome of patients undergoing transcatheter aortic valve implantation (TAVI) for a classification of chronic kidney disease (CKD) based on data from the FRANCE 2 registry. In daily TAVI practice, the assessment of classification of CKD stages before TAVI might be implicated in risk stratification. CKD 3b, 4 and 5 correlate with poor outcome and are considered a significant risk for TAVI. |
Funding
The FRANCE 2 registry was funded by a research grants from Edwards Lifesciences and Medtronic.
Conflict of interest statement
H. Eltchaninoff is a proctor for Edwards Lifesciences. E. Teiger is a proctor for Medtronic CoreValve. The other authors have no conflicts of interest to declare.

