
Abstract
Aims: There are no studies comparing transcatheter aortic valve implantation (TAVI) to conservative management in patients with chronic kidney disease stage 3-5 and severe aortic stenosis. We sought to compare the mortality rate and change in renal function in this patient population.
Methods and results: This was a single-centre retrospective cohort study that included all patients with chronic kidney disease stage 3-5 and severe aortic stenosis who underwent TAVI or were treated conservatively between 2010 and 2015. Three hundred and sixty patients were included (162 TAVI and 198 conservatively treated patients). Several statistical methods were used, including propensity score matching and inverse probability weighting. Mean follow-up was 1.9 years. Conservative management was associated with a hazard ratio of 3.95 (95% CI: 2.59-6.02) for mortality compared with TAVI. After one year there was a significant decrease in renal function in the control group (39.6±13.9 ml/min to 34.4±15.3 ml/min), but not in the TAVI group (41.7±13 ml/min to 42.9±14.5 ml/min) (p-value=0.001).
Conclusions: TAVI is associated with improved survival in patients with aortic stenosis and chronic kidney disease stage 3-5 compared to conservative management and protects from further decline in renal function up to one-year follow-up.
Abbreviations
AF: atrial fibrillation
AS: aortic stenosis
CKD: chronic kidney disease
eGFR: estimated glomerular filtration rate
ESRD: end-stage renal disease
HD: haemodialysis
IHD: ischaemic heart disease
SAVR: surgical aortic valve replacement
SCr: serum creatinine
TAVI: transcatheter aortic valve implantation
Introduction
Chronic kidney disease (CKD) affects up to 75% of aortic stenosis (AS) patients, increasing aortic valve disease prevalence and accelerating its progression1. In the CKD population, AS is associated with reduced survival2,3.
Up to 50% of AS patients with CKD are not referred to surgical aortic valve replacement (SAVR) due to excessive surgical risk4-6. Transcatheter aortic valve implantation (TAVI) has emerged as an alternative to SAVR in such patients7-10.
With few exceptions11-14, the current body of clinical evidence suggests that CKD increases post-TAVI mortality15-21.
Given their worse prognosis, the obvious question is whether the proven superiority of TAVI over conservative management in the overall severe AS population extends to CKD patients as well. At present, no study has compared mortality of patients with AS and CKD undergoing TAVI or managed conservatively. We sought to evaluate the effect of TAVI on survival and renal function in patients with stage 3-5 CKD and severe symptomatic AS compared to conservative management.
Methods
STUDY DESIGN
This was a retrospective cohort study.
STUDY POPULATION
We reviewed our centre’s echocardiography database for the period between January 2010 and December 2015 and identified patients with first diagnosis of severe symptomatic AS (mean aortic valve gradient ≥40 mmHg and symptoms of chest pain, dyspnoea or syncope). Patients who underwent SAVR (as documented in the patient’s electronic medical record) were excluded. We then divided the patients into two study groups – the TAVI and conservative management groups.
The patient selection process was as follows:
– TAVI group: patients were identified in our institution’s TAVI registry22. We used preoperative (<2 days) serum creatinine (SCr) values to calculate the baseline estimated glomerular filtration rate (eGFR) according to the CKD-EPI formula23. Patients with an eGFR >60 mL/min/1.73 m2 were excluded. All other patients were classified into four groups on the basis of baseline eGFR: 45-59 (CKD stage 3a), 30-44 (CKD stage 3b), 15-29 mL/min/1.73 m2 (CKD stage 4), and <15 mL/min/1.73 m2 or haemodialysis (CKD stage 5). Patients who had fewer than three measurements of SCr level in the year prior to the TAVI were excluded. eGFR was required to remain stable (within CKD stage) for at least two measurements during the year prior to inclusion.
– Conservative management group: we excluded all patients who did not have documented symptoms related to AS, as documented in either a hospital discharge paper or an outpatient clinic visit summary. Determination of eGFR and classification of patients into CKD subgroups was identical to that of the TAVI group.
In addition, we excluded patients with other significant valvular disease, patients with active metastatic neoplastic disease, and those with significant cognitive decline (as estimated by the attending physician in the cardiology/nephrology clinic). To prevent immortal time bias, control patients were included only if they survived the median time between the AS diagnosis and the TAVI procedure in the TAVI group.
FOLLOW-UP CREATININE VALUES
Follow-up creatinine levels were collected at approximately three months and one year after the diagnosis of symptomatic AS or the TAVI procedure from each patient’s electronic medical record.
ENDPOINTS
Our primary endpoint was overall mortality. The secondary endpoint included eGFR at three-month and one-year follow-up.
PROCEDURE
Candidates for TAVI were evaluated by the institutional Heart Team and the decision to perform TAVI was based on the patients’ clinical history, clinical status, anatomical suitability and geriatric assessment24-26.
STATISTICAL METHODS
Baseline patient characteristics are presented as means and standard deviations or median and interquartile range, as appropriate. Proportions were used for categorical variables. To compare baseline characteristics, the Student’s t-test/Mann-Whitney U test/Pearson χ2 test were used as appropriate.
We constructed a propensity score for prediction of the probability for referral to TAVI, using a logistic regression model with TAVI as outcome (Supplementary Table 1). The area under the receiver operating characteristic curve for this model was 0.78 (95% CI: 0.73-0.83).
We used several methodologies to evaluate the effect of TAVI on patient survival. First, we plotted Kaplan-Meier curves for mortality according to treatment group in the entire cohort and compared the risk of mortality with TAVI vs. conservative treatment using the log-rank test. To adjust for differences in baseline characteristics between the two groups and quantify the effect of TAVI on mortality, we used a multivariate Cox proportional hazards ratio model. A forward stepwise regression model was used with a p-value of 0.05 for inclusion and 0.1 for exclusion. The proportionality assumption was confirmed by assessing for significant interaction between the covariates and time.
Second, we stratified the patients into four groups according to propensity score quartiles. For this analysis we excluded patients below the 5th and above the 95th percentiles in order to reduce the effect of extreme propensity score values on the analysis. We used stratified Kaplan-Meier curves for mortality for each group and compared the results using the log-rank test for each stratum separately and across the propensity score strata combined.
Third, we assembled a propensity score-matched cohort with a 1:1 ratio of TAVI and conservative management patients, using a calliper of 0.02. A Kaplan-Meier survival curve was plotted with a log-rank test for significance level.
Finally, we used a generalised linear model with logit link function, with outcomes inversely weighted to each patient’s propensity score, to assess the odds ratio for survival up to five years at one-year intervals.
To identify characteristics associated with benefit/harm from TAVI, we performed interaction analysis by dichotomous subgroups categorised by age, gender, valve area, ejection fraction, eGFR, diabetes, atrial fibrillation (AF) and ischaemic heart disease (IHD).
Finally, we compared eGFR change between the groups during follow-up, using a repeated measures analysis of variance (ANOVA) to evaluate the interaction between TAVI and eGFR change over time at three months and one year compared to baseline.
Results
A total of 1,257 patients were diagnosed with severe AS at our institution during the study period. Thirty-four patients underwent SAVR; 317 of the remaining patients underwent TAVI. An additional 160 patients who underwent TAVI were not diagnosed at our centre. Of the total 477 patients who underwent TAVI, 198 had stable CKD stage 3-5 – these patients were included in the TAVI (intervention) group. Of the 901 patients who did not undergo SAVR or TAVI, 419 had stable CKD stage 3-5, 336 of whom had documented symptoms attributable to AS during the three months prior to the diagnosis. After applying all the pre-specified exclusion criteria, 162 patients were included in the conservative management (control) group. A detailed description of the patient inclusion/exclusion process for the study is shown in Supplementary Figure 1A and Supplementary Figure 1B.
PREPROCEDURAL AND OPERATIVE CHARACTERISTICS
Baseline demographic, echocardiographic and clinical characteristics of the study cohort by treatment group are presented in Table 1. Distribution of CKD stages and eGFR was similar between the TAVI and conservative management groups.
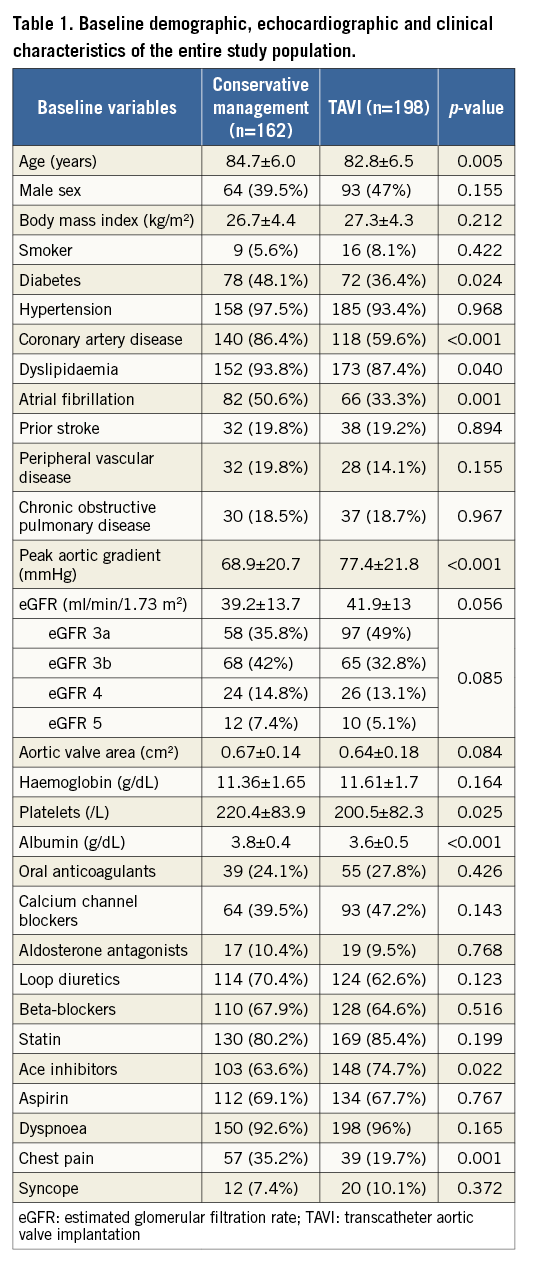
Compared with the TAVI group, patients in the conservative management group were older and had higher rates of diabetes, dyslipidaemia, IHD and AF, and complained more of chest pain. Patients in the TAVI group were more likely to be treated with ACE inhibitors compared with patients managed conservatively. There was no difference in left ventricular function between the two groups. However, patients in the TAVI group had a higher peak aortic valve gradient.
OVERALL SURVIVAL
After a mean follow-up of 1.9 years, the mortality rate was 32.7% (53/162) with TAVI vs. 49.5% (98/198) with conservative treatment (log-rank p<0.001). Using multivariate Cox analysis, conservative management was associated with a hazard ratio of 3.95 (95% CI: 2.59-6.02) for mortality compared with TAVI (Figure 1).
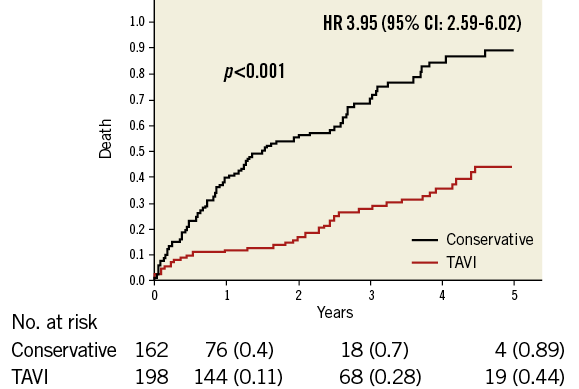
Figure 1. Effect of TAVI on mortality in the entire cohort. Kaplan-Meier analysis for overall survival of the entire cohort, comparing TAVI to conservative management. The HR is for the multivariate adjusted Cox model for overall mortality. HR: hazard ratio; TAVI: transcatheter aortic valve implantation
When stratified by propensity score quartiles, in each quartile TAVI was associated with better survival compared with conservative treatment (log-rank p=0.019, 0.004, <0.001 and 0.001 for quartiles 1 to 4, respectively), and the difference for the entire cohort across the strata was highly significant (p<0.001) (Supplementary Figure 2A-Supplementary Figure 2D).
PROPENSITY-MATCHED ANALYSIS
1:1 ratio propensity score matching yielded 80 patient pairs with no significant differences in baseline characteristics between TAVI and conservatively managed patients (Supplementary Table 2). A Kaplan-Meier survival curve showed significantly better survival for the TAVI group (p<0.001) (Figure 2).
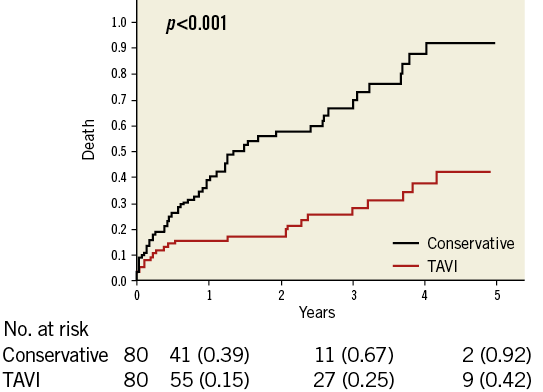
Figure 2. Effect of TAVI on mortality in the propensity-matched cohort. Kaplan-Meier analysis for overall survival in the propensity score-matched cohort, comparing TAVI to conservative management. TAVI: transcatheter aortic valve implantation
INVERSE PROBABILITY WEIGHTED ANALYSIS
One-year survival analysis included 300 patients (166 in the TAVI group and 134 in the conservatively managed group). Sixty patients were excluded for not having the required follow-up time period. Of the 300, 220 patients survived. The one-year mortality rate was significantly increased for patients in the conservative management group with 58 deaths (43.3%) vs. 22 deaths (13.3%) with TAVI (adjusted OR 4.48, 95% CI: 1.89-10.63). The second-, third-, fourth- and fifth-year mortality rates were also lower in the TAVI group compared with the conservative management group (Supplementary Table 3).
Subgroup analysis for valve area, left ventricular function, AF, eGFR, gender, diabetes and IHD for the outcome of one-year survival (Supplementary Figure 3) showed consistent results in all subgroups, apart from diabetes – which was associated with a significantly reduced survival advantage in the TAVI group (p for interaction=0.025).
CHANGE IN RENAL FUNCTION AT THREE MONTHS AND ONE YEAR
At three-month follow-up, eGFR in patients who underwent TAVI increased slightly from 41.7±13 ml/min to 42.9±15.5 ml/min. In the conservative management group, eGFR decreased from 39.6±13.9 ml/min to 37.2±14.2 ml/min. Using repeated measures ANOVA, this interaction was of borderline significance (p=0.049). At one-year follow-up, eGFR in the conservative management group decreased further (from 39.6±13.9 ml/min to 34.4±15.3 ml/min), while eGFR remained unchanged in the patients who underwent TAVI (41.7±13 ml/min and 42.9±14.5 ml/min). The interaction between eGFR change over time and treatment was highly significant (p=0.001) (Figure 3).
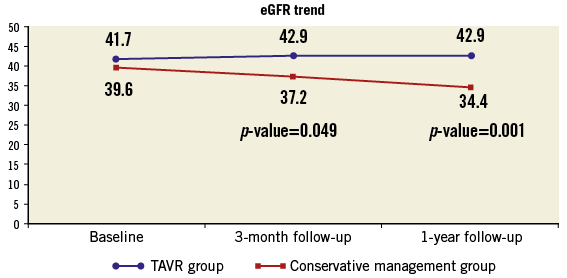
Figure 3. Trends in eGFR during one year of follow-up. eGFR as measured at baseline, three-month and one-year follow-up in the TAVI and control groups. eGFR: estimated glomerular filtration rate; TAVI: transcatheter aortic valve implantation
Discussion
Our results suggest that, for patients with stage 3-5 CKD and severe symptomatic AS, TAVI is associated with improved survival at one to five years of follow-up, compared to conservative management. In addition, patients who underwent TAVI showed a stable eGFR at one-year follow-up, whereas patients treated conservatively experienced a continued decline in eGFR.
Patients with advanced CKD are at increased risk of cardiovascular disease27. Besides traditional risk factors, patients with CKD have unique risk factors such as uraemic milieu, inflammation and abnormal vascular calcification, due to the profound effect of kidney failure on bone and mineral metabolism and the use of calcium-based phosphate binders28. AS is common in patients with CKD/end-stage renal disease (ESRD) and is associated with reduced survival. These disease states are interconnected, as CKD leads to calcification of the aortic valve, promoting worsening of AS, which, in turn, decreases kidney perfusion and contributes to the further decline in eGFR.
As opposed to earlier studies11-14, all recent studies including two meta-analyses16-21,29 reported that impaired renal function at baseline is a strong predictor of mortality following TAVI. Dumonteil et al20, in a cohort of 942 TAVI patients, demonstrated that patients with CKD who undergo TAVI have a higher risk profile and worse 30-day and one-year outcomes. Specifically, chronic haemodialysis and severe preprocedural CKD are independently associated with an increased risk of one-year mortality after TAVI. Yamamoto et al19, in a cohort of 642 TAVI patients, studied the prognostic effect of CKD category post TAVI. Patients were classified into four groups (CKD 1+2, CKD 3a, CKD 3b and CKD 4). The study showed an increase in 30-day and one-year mortality rates across the four groups. Allende et al18 divided 2,075 patients according to eGFR into four groups (CKD stage 1+2, CKD stage 3, CKD stage 4 and CKD stage 5). Advanced CKD (stage 4-5) was an independent predictor of 30-day major/life-threatening bleeding (p=0.001) and mortality (p=0.027), and late overall, cardiovascular and non-cardiovascular mortality (p<0.01 for all).
Physicians hesitate to refer CKD patients for TAVI because of the worse outcomes of patients with advanced CKD post TAVI compared to patients with normal kidney function, combined with the fact that undergoing TAVI is associated with exposure to several risk factors for acute kidney injury. This apprehension is due to the concern that the overall risk/benefit profile for TAVI may not be as favourable in the CKD population compared to the overall AS population. However, we should try to avoid making this comparison. When evaluating the risk/benefit profile for TAVI in the CKD population, one needs to compare the outcomes of patients with CKD and AS post TAVI, with those treated conservatively, and not to TAVI patients with no CKD. To the best of our knowledge, our study is the first to do so. Our results show the dismal prognosis of patients with both severe AS and advanced CKD (43% mortality at one year and over 95% mortality at five-year follow-up). Given these results, it is not surprising that TAVI was associated with a significantly improved survival in this population, when compared to conservative management. Our results suggest that CKD should not preclude severe AS patients from undergoing a thorough evaluation for eligibility for TAVI and show that, although their outcomes may be inferior to patients with normal renal function, their overall benefit from the procedure is significant.
Our results regarding the beneficial effect of TAVI on the preservation of renal function are in line with current knowledge. Although TAVI-treated patients are exposed to factors that put them at risk of acute kidney injury (exposure to contrast agent, hypotensive episodes, cholesterol embolisation), previous studies have shown that treating severe AS (with either SAVR or TAVI) is associated with improvement or at least preservation of renal function. Nguyen et al13 found that both SAVR and TAVI increased postoperative eGFR at discharge, and that TAVI resulted in a greater increase in eGFR compared to SAVR. Voigtländer et al30 demonstrated that in patients with normal renal function there was no significant change in eGFR after TAVI. Patients with moderately impaired renal function demonstrated a modest increase in eGFR, whereas the most prominent increase in eGFR was found in patients with severely impaired renal function. Beohar et al31 performed an analysis on CKD patients from the PARTNER 1 trial and showed that improved eGFR 30 days after TAVI did not change one-year outcomes while worsening eGFR was associated with increased mortality after one year. The main difference between our study and those mentioned above is that we evaluated both short- (three months) and intermediate-term (one year) eGFR change, as opposed to short-term changes only in previous studies. In addition, we included only advanced CKD patients, rather than overall AS patients, and compared the course of renal function following TAVI to that of patients treated conservatively. Our results are reassuring, suggesting that TAVI is safe in terms of renal outcomes in the CKD population. The preservation of renal function following TAVI, as opposed to the continued decline in the conservative management group, is probably due to an increase in renal perfusion. The latter may result from improved cardiac output with resolution of the obstruction to the left ventricle emptying, and from the alleviation of the chronic vasoconstrictive state of patients with severe AS, that serves as a compensatory mechanism to maintain blood pressure in the face of reduced effective ejection fraction32.
Limitations and strengths
Our study has several limitations. Its retrospective nature renders it prone to confounding, even with the use of statistical methods aimed at overcoming this issue. It is a single-centre study, hence the sample size is modest. In addition, since we do not have a formal assessment of frailty and cognitive function in the conservative management group, we cannot exclude the possibility of confounding due to between-group differences in these variables. However, serum albumin, one of the four components used to assess frailty, was actually higher in the conservative management group compared to the TAVI group, and patients who were judged to have significant cognitive impairment by their cardiologist/nephrologist were not included in the study.
On the other hand, our study has several strengths. It is the first to compare outcomes of conservative management with those of TAVI in a population of patients with both CKD and severe AS. We used several advanced statistical methods to reduce the possibility of confounding (propensity score matching, inverse probability weighting and Cox proportional hazards model), and our results were consistent and robust with all three methods. Our study is also unique in having a one-year follow-up regarding renal function post TAVI, compared to previous studies. Our study represents an “all comers” population that represents the patients seen in daily clinical practice by cardiologists, which reduces the inherent risk of selection bias that exists in randomised prospective trials.
Conclusions
Our study suggests that, for patients with CKD and severe AS, conservative management is associated with a dismal prognosis, while TAVI significantly improves short- and medium-term survival. TAVI also results in preservation of renal function up to one-year follow-up, as opposed to a significant decline in patients treated conservatively. These results should encourage both cardiologists and nephrologists to refrain from a priori excluding CKD patients from undergoing thorough assessment for TAVI eligibility. Further studies aimed at identifying characteristics of CKD patients that are associated with survival benefit following TAVI are needed, in order to improve clinical outcomes in this high-risk patient population.
| Impact on daily practice We hope that our results will encourage cardiologists and nephrologists to refrain from a priori excluding CKD patients from undergoing a thorough assessment for TAVI eligibility. |
Conflict of interest statement
The authors have no conflicts of interests to declare.
Supplementary data
Supplementary Figure 1. Patient selection process for inclusion in the study – conservative management group.
Supplementary Figure 2. Effect of TAVI on mortality by propensity score quartiles.
Supplementary Figure 3. Subgroup analysis for one-year survival.
Supplementary Table 1. Variables included in the final propensity score model.
Supplementary Table 2. Baseline characteristics of the propensity-matched cohort.
Supplementary Table 3. Inverse probability weighted mortality rates during long-term follow-up.
To read the full content of this article, please download the PDF.

