Abstract
Background: The association between renal morphological findings and changes in renal function in patients undergoing transcatheter aortic valve implantation (TAVI) is unexplored.
Aims: We aimed to investigate the association between renal morphological findings and changes in renal function in patients undergoing TAVI.
Methods: Among 283 consecutive patients undergoing TAVI between 2018 and 2021, the study sample included 224 patients. Renal morphological measurements were performed by preoperative multidetector computed tomography. Estimated glomerular filtration rate (eGFR) improvement and deterioration were defined as positive or negative changes in an eGFR of ≥10% one month after TAVI. The renal cortex thickness index was defined as the ratio of total renal cortex thickness to body surface area.
Results: The incidences of eGFR improvement and deterioration were 33.9% and 24.1%, respectively. The renal cortex thickness index had a significant correlation with changes in eGFR (r=0.34, p<0.01). The index of the area under the curve of renal cortex thickness for eGFR improvement and deterioration were 0.73 and 0.68, respectively. The cut-off values were 5.82 mm/m2 for eGFR improvement (odds ratio [OR]: 0.10; 95% confidence interval: 0.05-0.20; p<0.01) and 4.89 mm/m2 for eGFR deterioration (OR: 9.07; 95% confidence interval: 4.55-18.6; p<0.01).
Conclusions: The renal cortex thickness index was associated with changes in renal function in patients who underwent TAVI. Its measurements might be useful for predicting the renal function change in patients undergoing TAVI.
Introduction
Transcatheter aortic valve implantation (TAVI) is a well-established treatment option for severe aortic valve stenosis (AS)1, and most patients undergoing TAVI are of advanced age and have multiple comorbidities, such as chronic kidney disease (CKD)2. In previous reports, the estimated glomerular filtration rate (eGFR) improved in approximately 40% and deteriorated in approximately 25% of patients one month after TAVI34. Also, patients with deteriorated eGFR one month after TAVI have been reported to have a poor prognosis4. It is therefore important to predict the changes in renal function in patients undergoing TAVI.
The strong correlation between renal cortex thickness and renal function has been reported in an ultrasonography study5. Multi-detector computed tomography (MDCT) showed a more accurate measurement of cortex thickness of the kidney than ultrasonography6. However, how specific renal characteristics measured by MDCT are associated with eGFR improvement or deterioration in patients undergoing TAVI has not been explored. Therefore, we aimed to assess the association between the renal morphological findings measured by preoperative MDCT and the changes in renal function in patients undergoing TAVI.
Methods
STUDY SAMPLE AND PROTOCOL
This study is a retrospective, single-centre, observational study. Between March 2018 and March 2021, 283 consecutive patients with severe AS underwent TAVI in our hospital. After excluding patients with abnormal kidney morphology (defined as unilateral kidney, renal tumour, solitary cyst >4 cm in diameter, and polycystic kidney disease; n=30), poor CT image (n=19), death within one month after TAVI (n=4), and no serum creatinine measurement available two days, seven days, and one month after TAVI (n=6), 224 patients were analysed in this study (Figure 1)4. CKD patients were defined as those with an eGFR <60 mL/min/1.73 m2. Each eGFR was calculated using the CKD Epidemiology Collaboration equation7. The medical records contained all the laboratory results taken either during hospitalisation or in the community setting. We routinely measured the serum creatinine and eGFR on the day before TAVI, and two days, seven days, and one month after TAVI. The study was performed in accordance with the Declaration of Helsinki and the guidelines for epidemiological studies issued by the Ministry of Health, Labour, and Welfare of Japan. Informed consent was provided for the procedure and for subsequent data collection and analysis for research purposes.
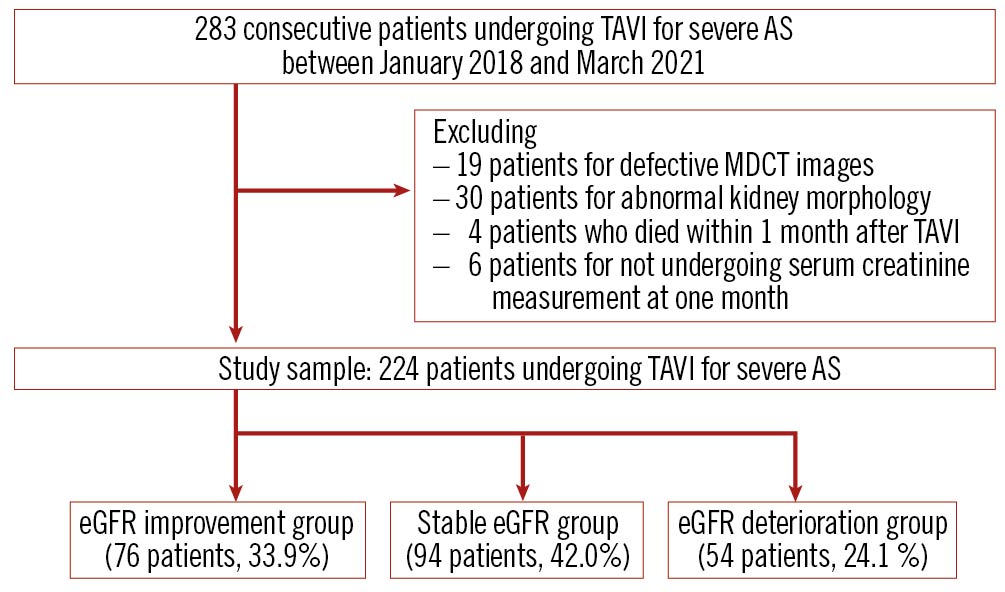
Figure 1. Study flow chart. AS: aortic valve stenosis; eGFR: estimated glomerular filtration rate; MDCT: multidetector computed tomography; TAVI: transcatheter aortic valve implantation.
MULTIDETECTOR COMPUTED TOMOGRAPHY ANALYSIS OF RENAL MORPHOLOGICAL FINDINGS
A 128-slice MDCT (SOMATOM Definition Flash; Siemens AG) was used to evaluate the renal morphological measurements in this study. Standard technical parameters were used. Contrast enhancement was achieved using a 60-120 mL non-ionic iodinated contrast agent (Iopamidol 370; Bayer Schering Pharma). The thickness of reconstructed images was 1.0 mm (1.0 mm increments) when imaging the thoracoabdominal aorta, including the kidneys. MDCT data sets were transferred to a dedicated workstation (Ziostation2; Ziosoft Inc.) for processing and renal morphological size measurement. Morphological measurements were performed as previously reported5. Renal length, as well as the cortex, medullary and parenchymal thickness were measured from longitudinal images. The renal cortex thickness was defined as the distance from the corticomedullary junction to the renal capsule, the medullary thickness was the distance from the sinus fat to the corticomedullary junction, and the parenchymal thickness was the distance from the sinus fat to the renal capsule. Cortex, medullary, and parenchymal thicknesses were averaged by measuring the thickest visible part of the kidney divided into three equal parts in the long axis. A three-dimensional planimetry system was utilised to obtain the total kidney volume (Figure 2). MDCT images were retrospectively segmented and reviewed by two independent individuals. All parameters were measured for bilateral kidneys, and the total values of the left and right kidneys were used for the analysis. The renal cortex thickness index was defined as the ratio of the total renal cortex thickness to body surface area (BSA).
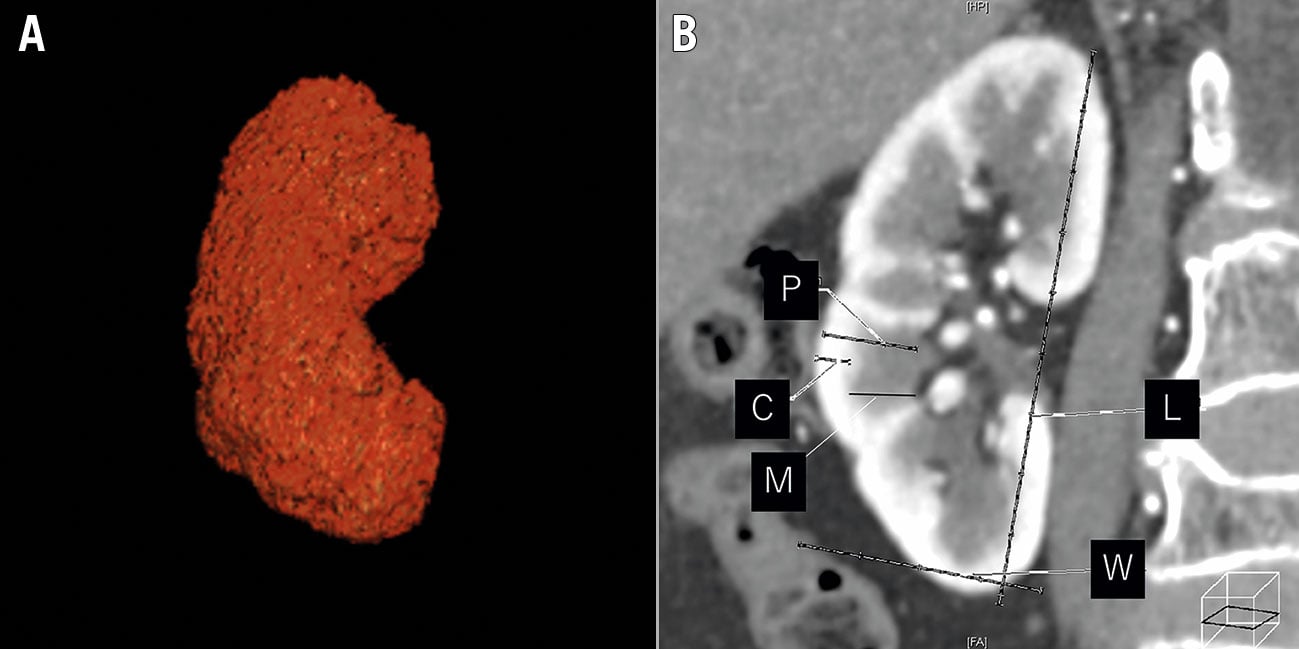
Figure 2. Three-dimensional (A) and longitudinal (B) multidetector computed tomography images of the measurements of the total kidney length, width, and thickness. C: cortex thickness; L: length; M: medullary thickness; P: parenchyma thickness; W: width.
TRANSCATHETER AORTIC VALVE IMPLANTATION
The procedure was performed under general anaesthesia with endotracheal intubation or local anaesthesia with conscious sedation. The patients were premedicated with at least one antiplatelet drug. TAVI was performed with either the self-expanding Evolut R/Pro (Medtronic) or the balloon-expandable SAPIEN XT/3 (Edwards Lifesciences) valves. The prosthesis size was determined using MDCT findings. The valves were delivered through femoral, apical, subclavian, brachiocephalic or transaortic approaches. Vasoconstricting agents were used for maintaining coronary perfusion pressure during periods of haemodynamic instability. An intravenous infusion of isotonic saline was continued for eight hours after the procedure, and the infusion volume was approximately 1,500 mL. Periprocedural AKI was based on a modified version of the Valve Academic Research Consortium 3 (VARC 3) criteria8 using the highest serum creatinine measurement (without assessing urine output) during the seven days after TAVI.
STEADY-STATE CHANGES IN EGFR ONE MONTH AFTER TAVI
Steady-state changes in eGFR after TAVI were defined as renal function improvement after TAVI (an increase of 10% or more in eGFR one month after TAVI compared with baseline), renal function deterioration after TAVI (a decrease of 10% or more in eGFR one month after TAVI compared with baseline), or stable renal function after TAVI (eGFR within 10% of baseline value one month after TAVI)4.
STATISTICAL ANALYSIS
Categorical variables were compared using the chi-square test. Continuous variables were expressed as mean±standard deviation and compared using the Student’s t-test or Wilcoxon rank-sum test based on the distributions. Data were presented as mean±standard deviation for continuous variables. These measurements were subsequently compared between patients with eGFR improvement, those with stable eGFR, and those with eGFR deterioration. Patients with stable eGFR served as the reference group in all multivariate logistic regression models. The initial models used variables with an overall p-value ≤0.10 from the baseline patient characteristics, procedural results and renal morphology measurements. Using the variables, a multivariate logistic regression model was built to identify the risk factors for the renal function changes. The correlations of morphological parameters with renal function were analysed using Pearson’s correlation coefficient and Spearman’s rank correlation coefficient. A receiver operating characteristic (ROC) curve was used to assess the ability of morphological parameters to predict eGFR improvement or deterioration. The area under the curve (AUC) value was used as an indicator of predictive accuracy. Numbers with relative percentages are reported for categorical variables. Two-tailed p-values <0.05 were considered to have a statistical significance. All statistical analyses were performed using JMP 9.0 (SAS Institute).
Results
BASELINE PATIENT CHARACTERISTICS AND PROCEDURAL RESULTS
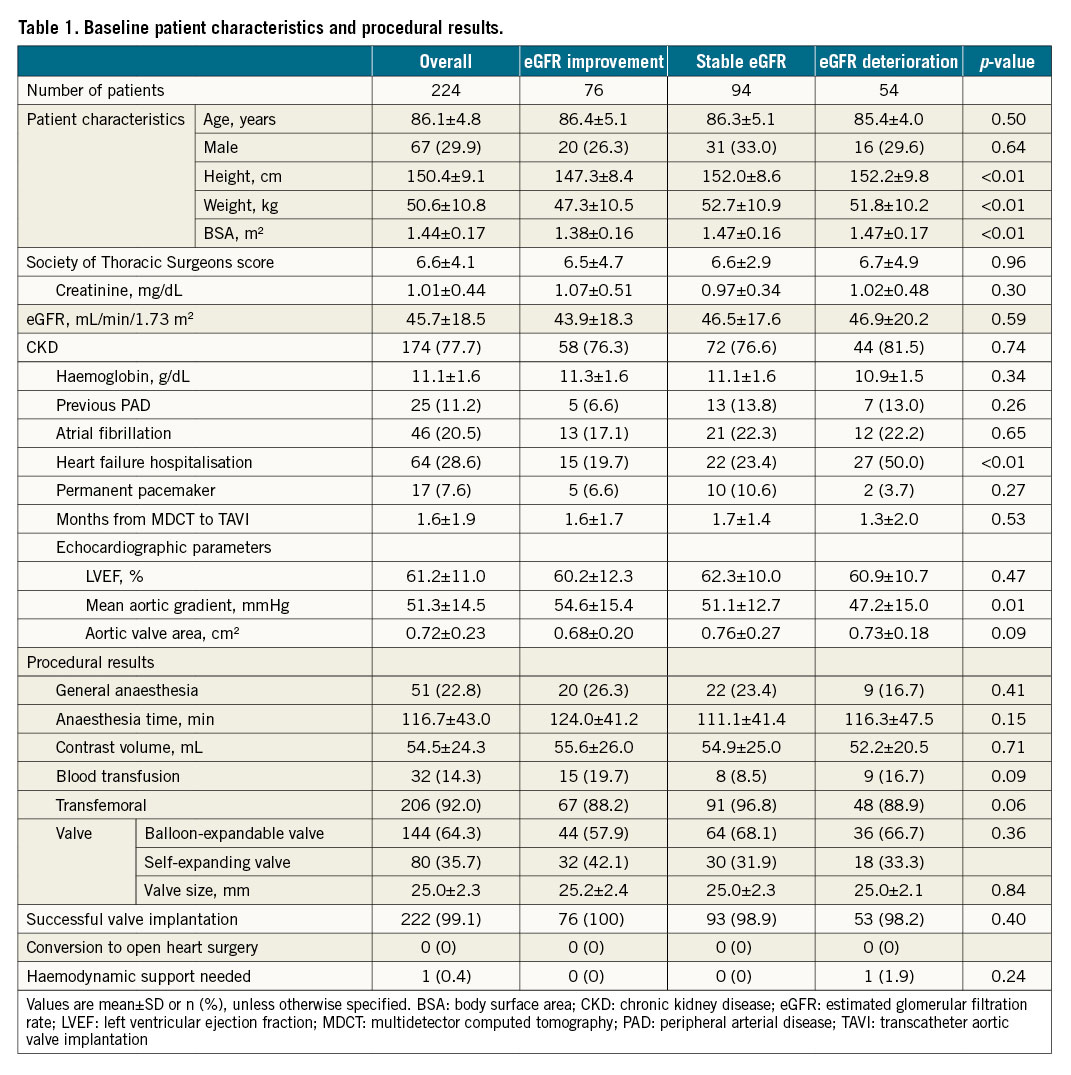
The baseline patient characteristics and procedural results are summarised in Table 1 and Supplementary Table 1. The mean age was 86.1±4.8 years, and the mean Society of Thoracic Surgeons (STS) score was 6.6±4.1. The mean serum creatinine and eGFR were 1.01±0.44 mg/dL and 45.7±18.5 mL/min/1.73 m2, respectively. In this study, the incidences of eGFR improvement or deterioration one month after TAVI were 33.9% and 24.1%, respectively. The procedural contrast volume was equivalent for eGFR improvement, stable GFR and eGFR deterioration groups. Post-procedural results are summarised in Supplementary Table 2. Periprocedural AKI occurred in 40 patients (17.9%).
RENAL MORPHOLOGY MEASUREMENTS AND SERIAL CHANGES IN RENAL FUNCTION THROUGH TAVI TREATMENT
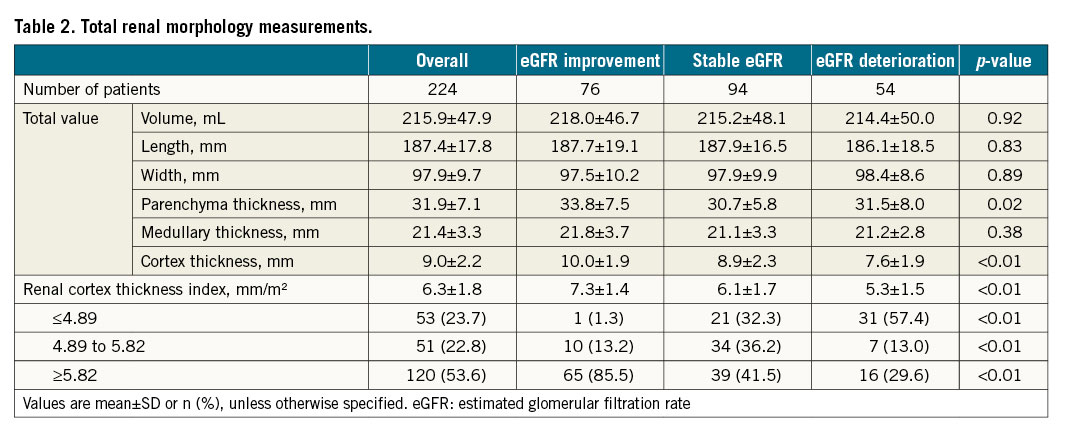
Renal morphology measurements are summarised in Table 2 and Supplementary Table 3. The left and right kidneys had a total volume, length and width of 215.9 mL, 187.4 mm and 97.9 mm, respectively. The total parenchymal, medullary and cortex thickness of both kidneys were 31.9 mm, 21.4 mm and 9.0 mm, respectively. The proportions of the renal cortex thickness index were 23.7% (4.89 mm/m2 or less), 22.8% (from 4.89 to 5.82 mm/m2) and 53.6% (5.82 mm/m2 or more), respectively.
Supplementary Table 4 summarises the baseline and serial changes in the renal function data in patients after TAVI. The variations of serum creatinine and eGFR two days, seven days and one month after TAVI were −0.05±0.31 mg/dL (100.8±28.1%) and 3.3±12.7 mL/min/1.73 m2 (109.3±27.8%), −0.04±0.25 mg/dL (102.5±24.0%) and 3.1±12.1 mL/min/1.73 m2 (109.2±27.2%), and 0.01±0.26 mg/dL (102.3±25.7%) and 0.7±11.8 mL/min/1.73 m2 (103.4±25.2%), respectively. The percent changes in eGFR two days, seven days and one month after TAVI from baseline in each group are shown in Figure 3.
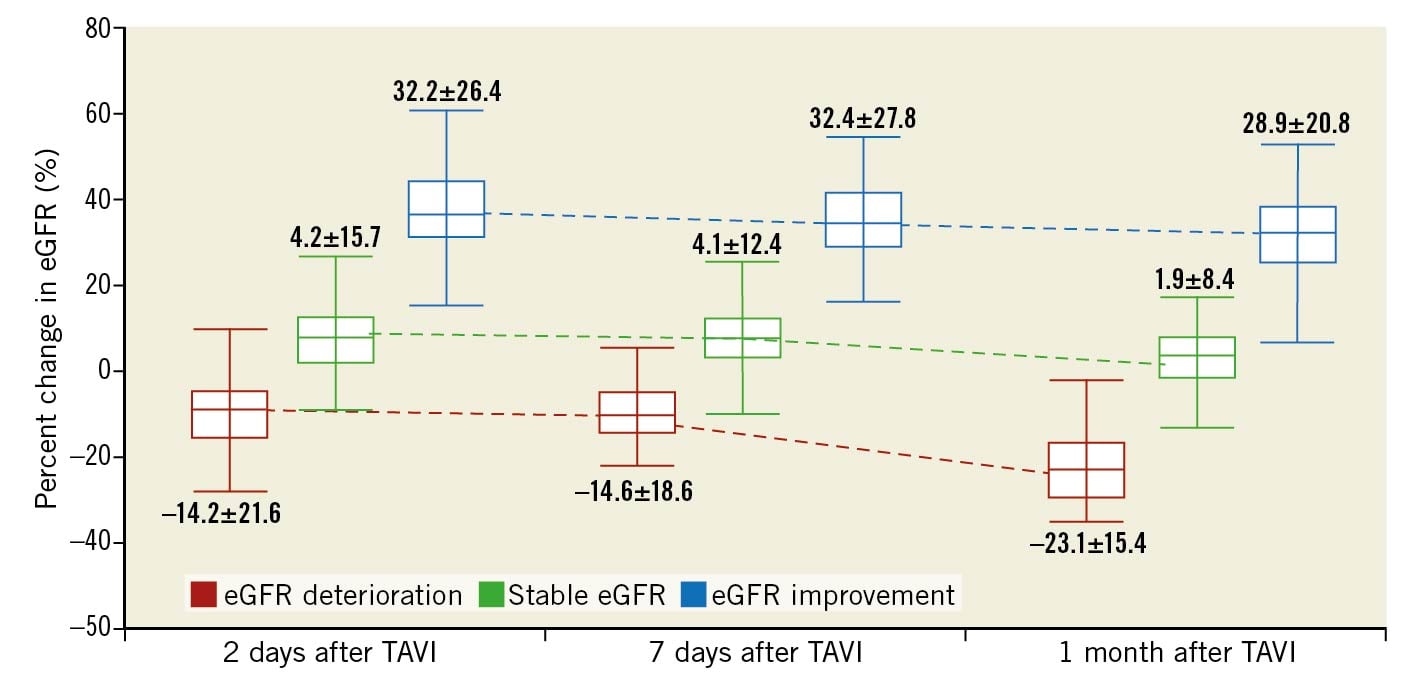
Figure 3. Percent change in eGFR two days, seven days and one month after TAVI from baseline. eGFR; estimated glomerular filtration rate; TAVI: transcatheter aortic valve implantation.
Overall, renal function in 115 patients (51.3%) one month after TAVI was better than that before TAVI. Figure 4 shows the eGFR at baseline and one month after TAVI for each group. The representative cases of patients with eGFR improvement and deterioration are demonstrated in Figure 5.
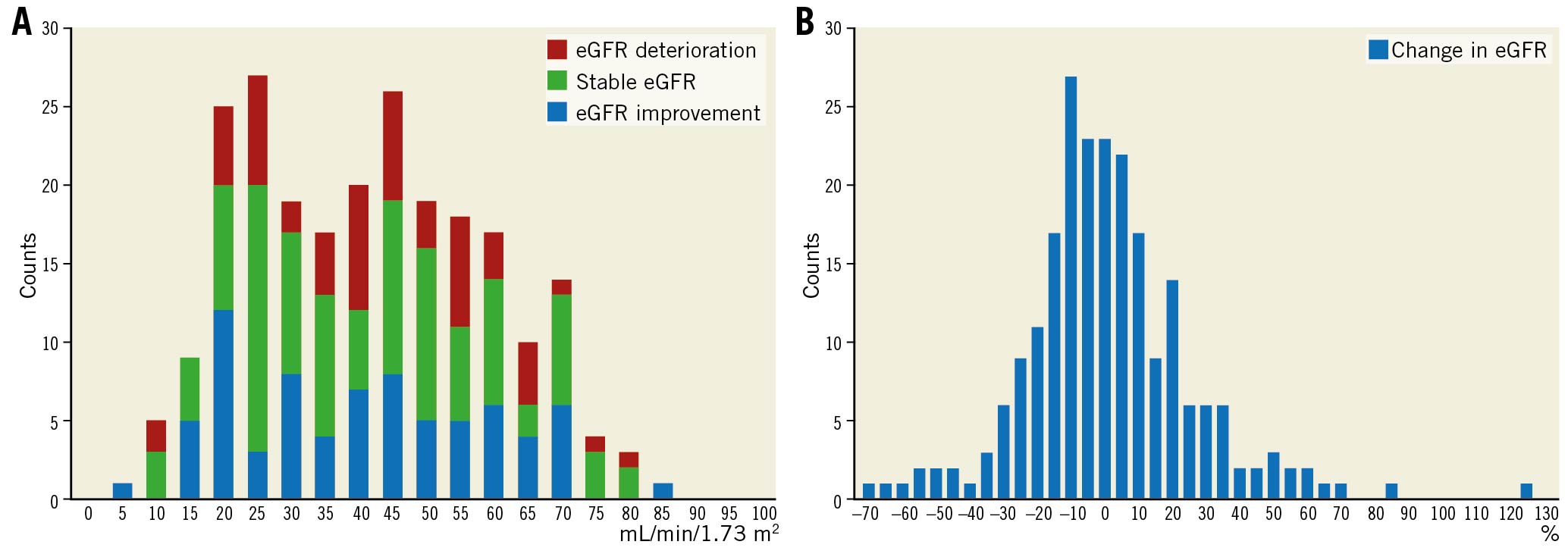
Figure 4. One-month eGFR changes. Relationship between baseline eGFR and changes in eGFR one month after TAVI (A) and the distribution of the state change in eGFR one month after TAVI (B) for each group. eGFR: estimated glomerular filtration rate; TAVI: transcatheter aortic valve implantation.
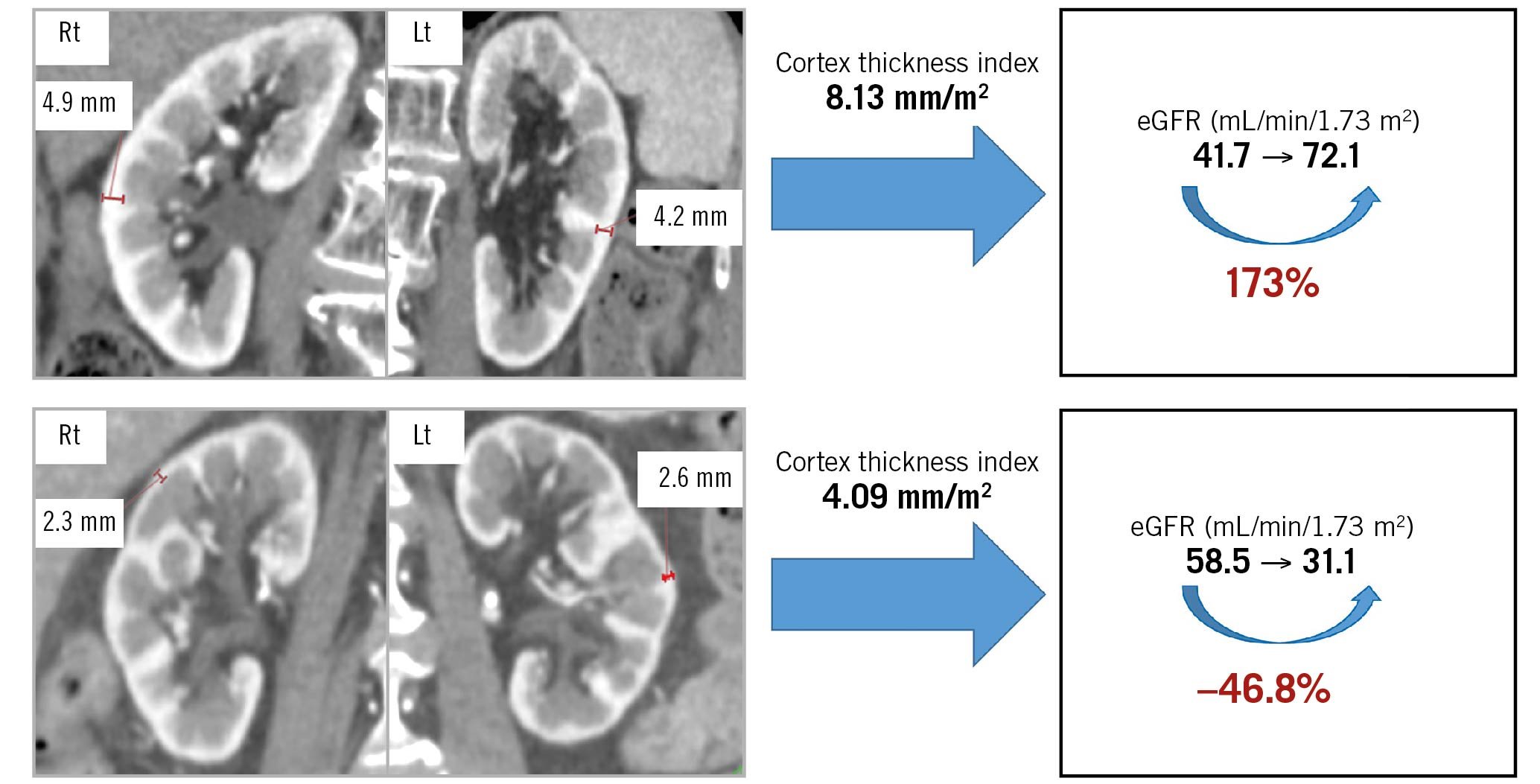
Figure 5. Improvement and deterioration of eGFR after TAVI. Upper panels: A representative case of eGFR improvement after TAVI. The total value of the renal cortex thickness index was 8.13 mm/m2. The eGFR value changed from 41.7 mL/min/1.73 m2 before to 72.1 mL/min/1.73 m2 one month after TAVI, a change of 173%. Lower panels: A representative case of eGFR deterioration after TAVI. The total value of the renal cortex thickness index was 4.09 mm/m2. The eGFR value changed from 58.5 mL/min/1.73 m2 before to 31.1 mL/min/1.73 m2 one month after TAVI, a change of −46.5%. eGFR: estimated glomerular filtration rate; Lt: left; Rt: right; TAVI: transcatheter aortic valve implantation.
PREDICTORS OF EGFR IMPROVEMENT AND DETERIORATION AFTER TAVI
The correlations between the changes in eGFR and renal morphological parameters detected by MDCT are shown in Figure 6. The renal cortex thickness index showed a significantly low correlation with the change in eGFR (r=0.34, p<0.01). The Central illustration shows the ROC curve of the renal cortex thickness index for predicting eGFR improvement and deterioration. The renal cortex thickness index exhibited a sensitivity and a specificity of 86.8% and 58.5% for predicting eGFR improvement and 57.4% and 79.8% for predicting eGFR deterioration, respectively. The ROC-AUC for eGFR improvement was 0.73, and the cut-off value was 5.82 mm/m2 (odds ratio [OR]: 0.10; 95% confidence interval [CI]: 0.05-0.20; p<0.01). Furthermore, the ROC-AUC for eGFR deterioration was 0.68, and the cut-off value was 4.89 mm/m2 (OR: 9.07; 95% CI: 4.55-18.6; p<0.01).
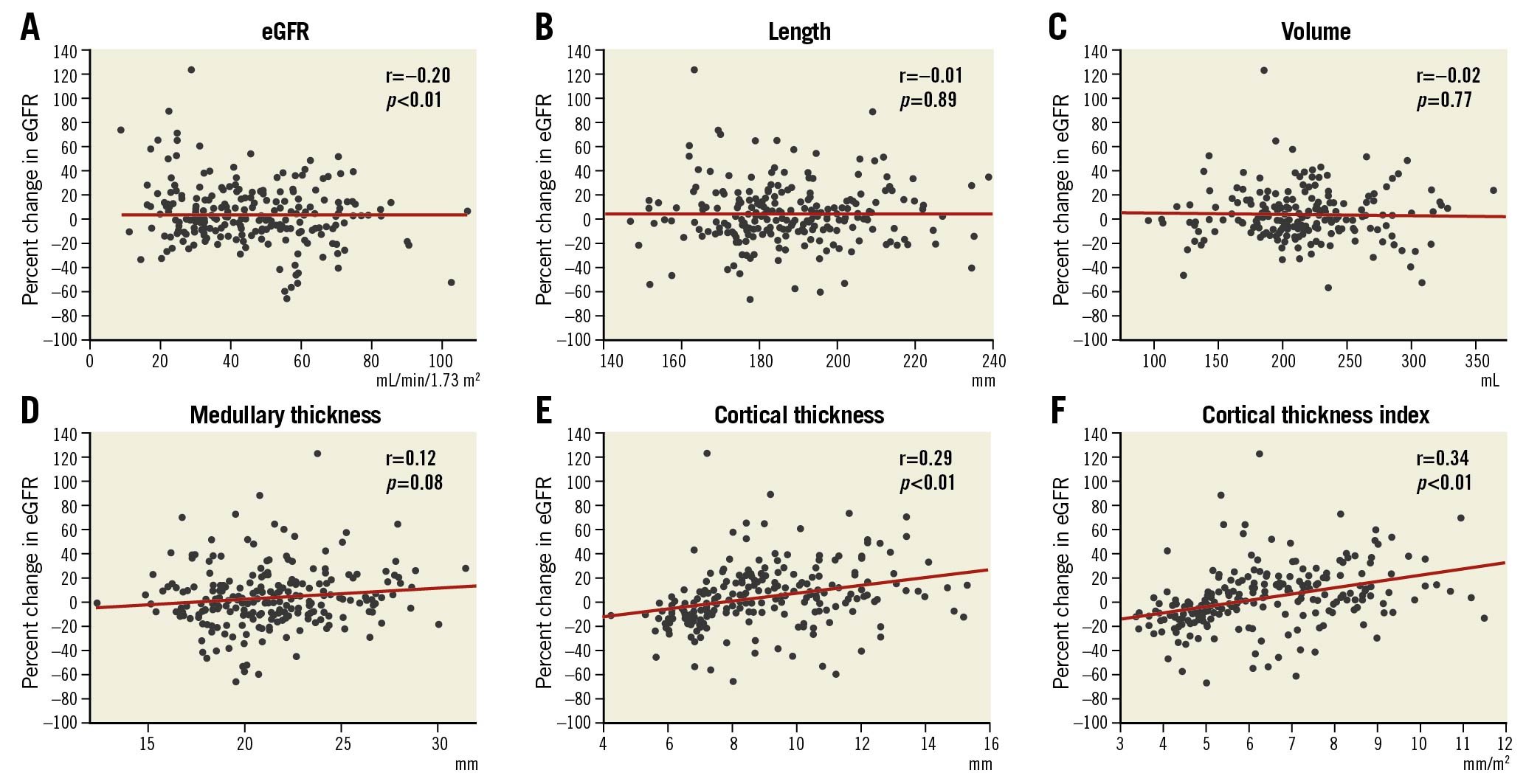
Figure 6. Correlation of morphological parameters by MDCT and decline in renal function one month after TAVI. Scatter plots comparing the percent change in eGFR with baseline eGFR (A), kidney length (B), volume (C), medullary thickness (D), cortex thickness (E), and cortex thickness index (F). eGFR: estimated glomerular filtration rate; TAVI: transcatheter aortic valve implantation.
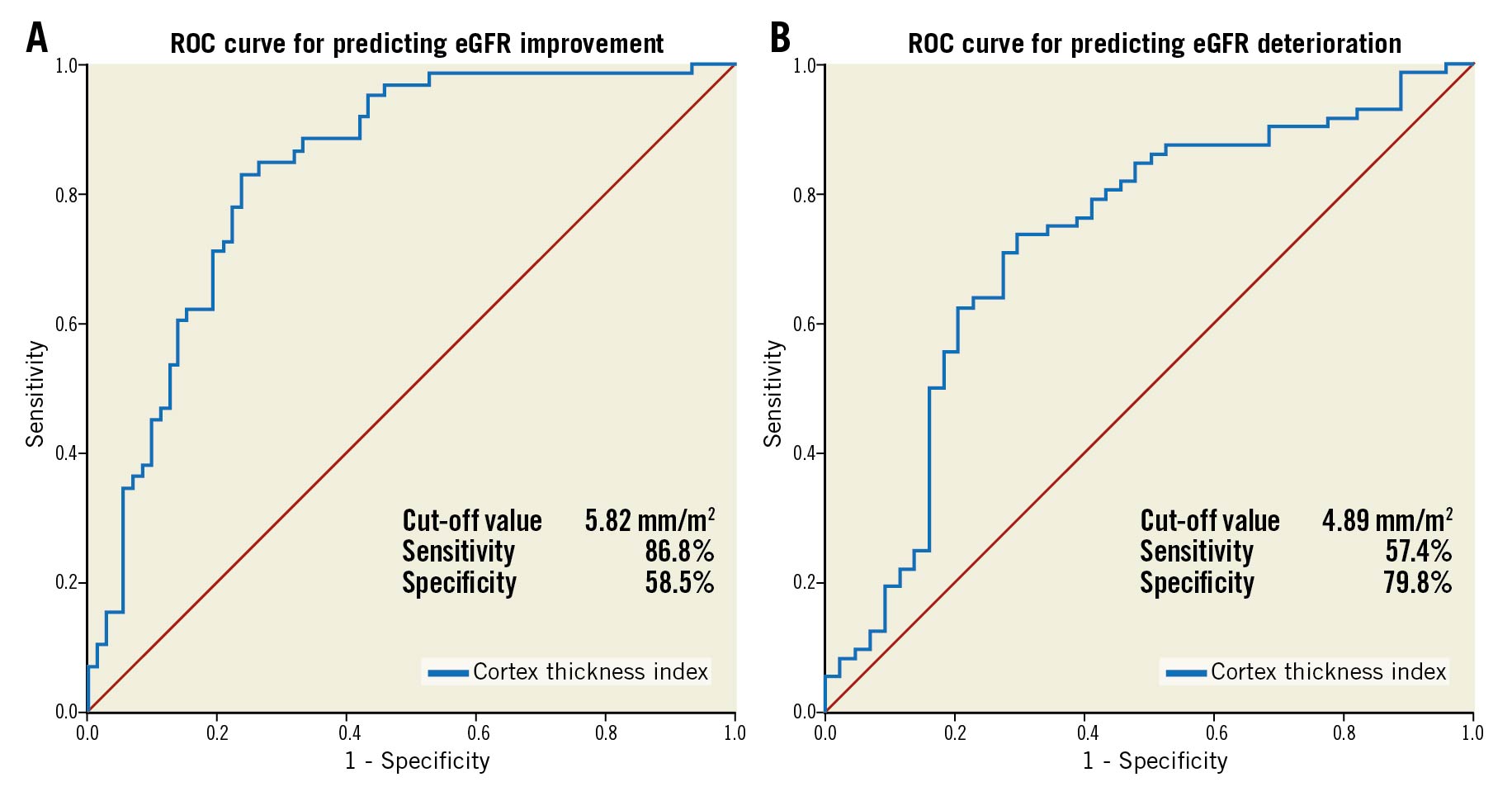
Central illustration. ROC curve of the total value of renal cortex thickness index for predicting eGFR improvement and deterioration (B). The renal cortex thickness index exhibited a sensitivity and specificity of 86.8% and 58.5%, respectively, for predicting eGFR improvement. A) For predicting eGFR improvement, the ROC-AUC was 0.73, and the cut-off value was 5.82 mm/m2 (OR: 0.10; 95% confidence interval: 0.05-0.20; p<0.01). The renal cortex thickness index exhibited a sensitivity and a specificity of 57.4% and 79.8%, respectively, for predicting eGFR deterioration. B) For predicting eGFR deterioration, the ROC-AUC was 0.68, and the cut-off value was 4.89 mm/m2 (OR: 9.07; 95% confidence interval: 4.55-18.6; p<0.01). AUC: area under the curve; eGFR: estimated glomerular filtration rate; ROC: receiver operating characteristic.
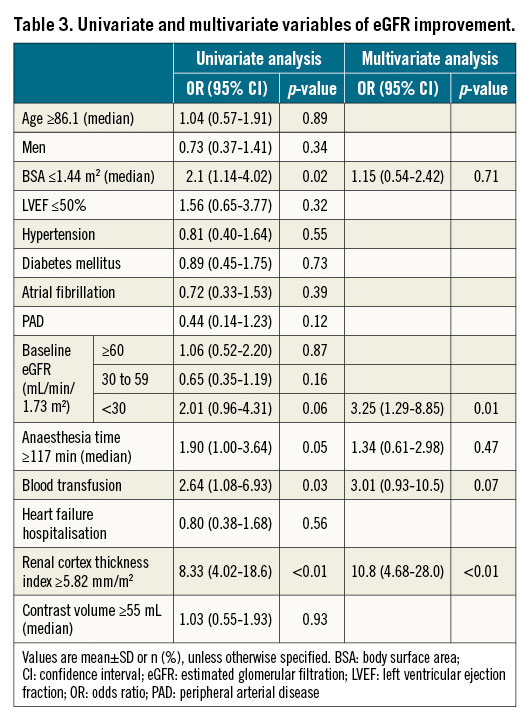
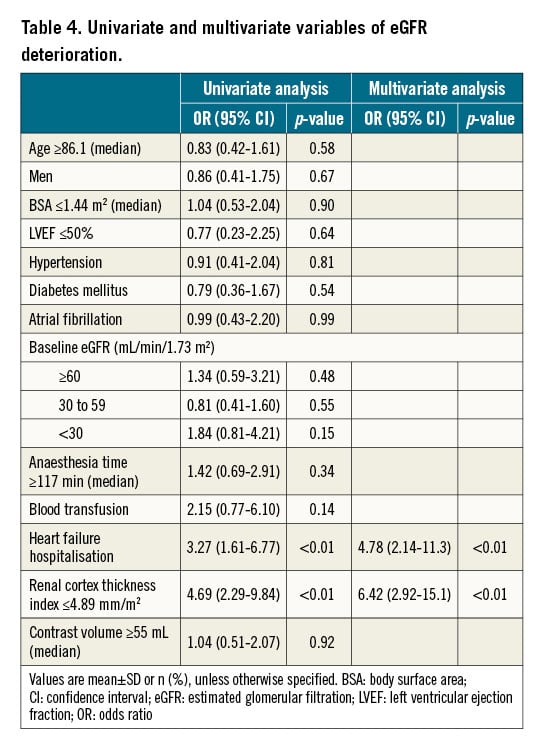
Univariate and multivariate variables in patients with eGFR improvement are shown in Table 3. In the multivariate analysis, the renal cortex thickness index ≥5.82 mm/m2 (OR: 10.8; 95% CI: 4.68-28.0; p<0.01) and eGFR <30 mL/min/1.73 m2 (OR: 3.25; 95% CI: 1.29-8.85; p=0.01) were independent predictors of eGFR improvement in patients receiving TAVI. Univariate and multivariate variables in patients with eGFR deterioration are shown in Table 4. In the multivariate analysis, the renal cortex thickness index ≤4.89 mm/m2 (adjusted OR: 6.42; 95% CI: 2.92-15.1; p<0.01) and heart failure hospitalisation within three months before TAVI (adjusted OR: 4.78; 95% CI: 2.14-11.3; p<0.01) were independent predictors of eGFR deterioration in patients receiving TAVI.
RENAL FUNCTION CHANGE AFTER TAVI IN NON-CKD AND CKD PATIENTS
The incidences of eGFR improvement and deterioration in non-CKD patients one month after TAVI were 36.0% and 20.0%, respectively. The correlations between the changes in eGFR and renal morphological parameters detected by MDCT in non-CKD patients are shown in Supplementary Figure 1. The renal cortex thickness index showed significant correlation with the change in eGFR (r=0.46, p<0.01).
The incidences of eGFR improvement and deterioration in CKD patients one month after TAVI were 33.3% and 25.3%, respectively. The correlations between the changes in eGFR and renal morphological parameters detected by MDCT in CKD patients are shown in Supplementary Figure 2. The renal cortex thickness index showed significant correlation with the change in eGFR (r=0.50, p<0.01).
There was significant interaction between the CKD and non-CKD patients and the renal cortex thickness indexes on both eGFR improvement (p=0.03 for interaction) and deterioration (p<0.01 for interaction).
REPRODUCIBILITY OF RESULTS OF RENAL CORTEX THICKNESS MEASUREMENT
Intra- and inter-observer variability for the total value of renal cortex thickness was analysed in 25 randomly selected patients. The mean difference±SD and 95% upper to lower limits of agreement were −0.02±0.25 mm (−0.53 to 0.48 mm) and −0.05±0.20 mm (−0.45 to 0.35 mm) for the intra- and inter-observer variability, respectively.
Discussion
The main findings of this study were as follows: first, the incidences of eGFR improvement and deterioration one month after TAVI were 33.9% and 24.1%, respectively. Second, the renal cortex thickness index was an independent predictor of eGFR improvement and deterioration in patients receiving TAVI. There was a significant interaction for CKD patients between the renal cortex thickness index and the renal function change. Third, heart failure hospitalisation within three months before TAVI was an independent predictor of eGFR deterioration in patients receiving TAVI.
INCIDENCES OF EGFR IMPROVEMENT AND DETERIORATION
Witberg et al used renal function 30 days after TAVI to evaluate renal function change through TAVI, because this timepoint is more reflective of stable renal function after TAVI. In the above-mentioned study, eGFR improvement and deterioration had incidence rates of 36.8% and 26.1%, respectively, and eGFR improvement occurred more frequently than eGFR deterioration4. In this study, the incidence of eGFR improvement was also higher than that of eGFR deterioration, although it was slightly lower than those of other studies34. This might be based on the difference between baseline eGFR and race specificity. Still, a certain number of patients can acquire renal function recovery by receiving TAVI.
CORRELATION BETWEEN RENAL CORTEX THICKNESS AND RENAL FUNCTION CHANGE
Recent studies have demonstrated the usefulness of renal morphological assessment in managing CKD91011. The kidney is composed of the cortex, medulla, and non-parenchymal tissues such as sinus fat and calyces, and the renal cortex thickness was only positively correlated with renal function change because the collecting ducts that control the renal filtration function are distributed in the cortex56. In addition, it was reported that the renal volume correlates with BSA12, and we therefore speculated that using the ratio of the total renal cortex thickness to BSA would be more suitable than only using the renal cortex thickness.
In this study, the renal cortex thickness index was correlated with the renal function change one month after TAVI in both non-CKD and CKD patients. In particular, there was a significant interaction for CKD patients between the indexes and the renal function change. Non-CKD patients had less variation in the renal cortex thickness index than CKD patients. In all non-CKD patients, the renal cortex was thicker than 5.82 mm/m2. It might be difficult to set the index to predict the renal function change for non-CKD patients in this relatively small sample size. Further larger samples are needed to detect the predictive ability of the renal cortex thickness indexes for non-CKD-patients.
PREDICTORS OF EGFR IMPROVEMENT AND DETERIORATION AT ONE MONTH
The reason for improvement in eGFR in patients after TAVI is unclear. There might be an interaction between AS and renal dysfunction. Previous studies showed that the haemodynamic effects of AS cause cardiorenal syndrome13 and that renal dysfunction promotes AS progression14. Treating AS via TAVI may break this vicious cycle and lead to renal improvement as well as improved cardiac function4. In this study, renal function in patients with a larger cortex thickness index improved after TAVI. The patients with a preserved collecting duct volume, especially those with CKD, might benefit from TAVI. It has been reported that the use of β-blockers, age, left ventricular mass and non-smoking were predictors of eGFR improvement in patients after TAVI315, although they were not significant predictors of eGFR improvement in this study. The rate of administration of β-blockers was relatively low and the average age was older in the above-mentioned studies than in this study, and this may have influenced the results.
It has been reported that the predictors of eGFR deterioration in patients after TAVI included STS score, age, left ventricular mass, non-smoking, and AKI at discharge34. In this study, eGFR deterioration was more likely to occur in patients with a low cortex thickness index. Although the reasons are unclear, renal function in patients without preserved collecting duct volumes might more easily be affected by other factors, even if their cardiac output increased after TAVI. In a previous study, 18-40% of patients experienced worsening of renal function in the context of acute decompensated heart failure16, and this might be due to other factors, such as medications and renal congestion. Furthermore, several risk factors have been associated with worsening renal failure, and one of the risk factors is a history of heart failure hospitalisation17. As previously reported, heart failure hospitalisation in the three months before TAVI was an independent predictor of eGFR deterioration in patients receiving TAVI in this study. The TAVI procedure is effective in stabilising AS and can reduce the future risk of heart failure rehospitalisation. However, deteriorating eGFR in patients one month after TAVI has been associated with increased mortality34. The prevention strategies for eGFR deterioration in patients with a recent history of heart failure hospitalisation are particularly crucial to improve patient prognosis after a successful TAVI.
The measurement of renal cortex thickness detected by MDCT is possible without additional imaging because the bilateral kidneys are included in the imaging range for TAVI screening. Further prospective studies with larger samples are needed, however. This novel index might become an indication for better clinical decision making for severe AS patients before receiving TAVI.
Limitations
There are four major limitations in this study. First, this is a retrospective, single-centre, observational study with a relatively small study population, therefore it is not free from bias. However, this study’s samples were large enough to validate the difference of the renal cortex thickness and changes in renal function. Second, peri-TAVI management was at the discretion of each physician, which may have affected the incidence of AKI and the improvement and deterioration in eGFR. Third, improvements in cardiac output, such as recovery in left ventricular function and changes in renal arterial blood flow, were not observed. Finally, there is a time divergence between the timing of MDCT and the measurement of baseline data. However, the renal cortex thickness immediately before TAVI would be almost unchanged.
Conclusions
The measurement of the renal cortex thickness detected by preoperative MDCT might be useful for predicting the renal function change in patients with severe AS undergoing TAVI, especially in those having CKD.
Impact on daily practice
Without performing additional imaging procedures in patients before TAVI, the renal cortex thickness index can be measured using preoperative MDCT. Its measurements might be useful in predicting the renal function change at one month in patients with severe AS undergoing TAVI.
Acknowledgments
The authors are grateful to the staff members of the cardiac catheterisation laboratory, and Miho Kobayashi, Makiko Kanaike, Takako Yukiyoshi, and Masashi Takata for their assistance with the manuscript.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

