
Abstract
Aims: Embolisation of atheromatous debris during catheter manipulation is considered to underlie acute cerebrovascular events (CVE) after transcatheter aortic valve implantation (TAVI). However, the relationship between aorta atheroma burden and acute CVE after TAVI has not been established. We investigated the impact of aorta atheroma burden on acute CVE.
Methods and results: Preoperative multislice computed tomographic (MSCT) images in 278 patients receiving TAVI were analysed. Total atheroma volume (TAV) was calculated by measuring aorta vessel and lumen areas in every 1 mm cross-sectional image. Acute CVE was observed in 16 patients. Patients having acute CVE were more likely to have a prior CVE (p=0.002), and to exhibit greater TAV in the ascending aorta (12.8±3.5 vs. 7.0±2.1 cm3, p<0.001) and the aortic arch (3.1±1.6 vs. 1.2±0.2 cm3, p<0.001). TAV in the ascending aorta >10.3 cm3 and in the aortic arch >2.9 cm3 predicted acute CVE. The incidence of acute CVE was highest (36.4%) if patients had a prior CVE and TAV in the ascending aorta and the aortic arch above cut-offs.
Conclusions: Patients with acute CVE after TAVI had greater aorta atheroma burden. Our findings might underscore preoperative MSCT analysis of aorta atherosclerosis to identify high-risk patients for acute CVE, who might require an embolic protection device during TAVI.
Abbreviations
CVE: cerebrovascular events
MSCT: multislice computed tomography
TAV: total atheroma volume
TAVarch: total atheroma volume in the aortic arch
TAVascending: total atheroma volume in the ascending aorta
TAVI: transcatheter aortic valve implantation
Introduction
Despite the clinical efficacy of transcatheter aortic valve implantation (TAVI) in inoperable or high surgical risk patients with severe symptomatic aortic valve disease1, the PARTNER (Placement of Aortic Transcatheter Valves) trial has raised safety concerns by showing a 30-day stroke rate of 6.7% in inoperable patients treated with a balloon-expandable bovine pericardial valve2. These cerebrovascular events (CVE) were more frequent after TAVI compared to surgical procedure in high-risk patients (5.5 vs. 2.4%, p=0.04)3. In another clinical trial comparing a self-expanding porcine pericardial valve with surgical aortic valve replacement, the stroke rate was 4.9% and 6.2% at 30 days (p=0.46) in the TAVI and surgical groups, respectively4. Given the morbidity and mortality associated with CVE, it is necessary to establish better pre-procedural assessment and use of effective risk reduction strategies.
CVE after TAVI have been shown to occur most frequently in the first 24 hours5,6. The interventional procedure, through the manipulation of guidewires and catheters for example, is considered to induce embolism of debris from the aortic wall or aortic valve. Additionally, it has been reported that scraping of aortic plaques occurs in >50% of percutaneous cardiac interventions and is more frequent when larger catheters are used7,8. Since large diameter devices (≥18 Fr) are currently required for TAVI, we hypothesised that the presence of a larger amount of atheroma within the aorta might be associated with acute CVE after the procedure.
Preoperative multislice computed tomography (MSCT) imaging has become an important tool in the workup of patients who are being considered for TAVI9. This modality also enables visualisation of the burden of aortic atherosclerosis. The purpose of this analysis was to quantify aortic atheroma burden in patients with severe aortic stenosis undergoing TAVI and its association with periprocedural CVE.
Methods
STUDY POPULATION
Two hundred and seventy-eight (278) consecutive patients with symptomatic severe aortic valve stenosis undergoing TAVI at the Royal Adelaide Hospital between August 2009 and July 2015 were analysed. Eligibility for TAVI was based on the consensus of a multidisciplinary team composed of interventional cardiologists and cardiac surgeons. The current study was approved by the institutional review committee. All patients consented to the performance and analysis of the CT data set prior to the TAVI procedure and gave informed consent.
TAVI PROCEDURES
Technical aspects of TAVI procedures with the use of a balloon-expandable or a self-expanding stent valve have been described in detail previously10,11. After conducting valvuloplasty of the aortic valve with a 20 to 23 mm balloon catheter under right ventricular pacing, one of the following four TAVI systems was utilised: CoreValve® (Medtronic, Minneapolis, MN, USA), SAPIEN XT (Edwards Lifesciences, Irvine, CA, USA), Lotus™ (Boston Scientific, Marlborough, MA, USA) or Portico™ (St. Jude Medical, St. Paul, MN, USA).
Patients were treated with aspirin and clopidogrel the day before TAVI. Patients receiving oral anticoagulation therapy were instructed to stop three days before the procedure, and were switched to continuous intravenous unfractionated heparin administration. During the procedure, intravenous unfractionated heparin was administered to achieve an activated clotting time >250 seconds. After the procedure, aspirin was continued indefinitely, whereas clopidogrel was discontinued after six months.
PREOPERATIVE MSCT IMAGING
A plain and contrast-enhanced MSCT was performed in all patients by using a 128 detector system (Siemens Definition AS+; Siemens Medical Solutions, Erlangen, Germany). Sublingual nitroglycerine and a beta-blocker were administered prior to MSCT imaging. After plain MSCT images were obtained, contrast-enhanced ones were acquired during injection of non-ionic iodinated contrast agent (Ultravist 370™; Bayer Healthcare, New York, NY, USA). Retrospectively, ECG-gated data acquisition was used with 128×0.6 mm collimation, scan pitch of 0.18 and a gantry rotation time of 300 ms.
MEASUREMENTS OF AORTA ATHEROMA BURDEN AND CALCIUM VOLUME AT AORTIC VALVE COMPLEX
Acquired MSCT images were analysed by a commercially available software (3mensio Structural Heart, version 5.1; Pie Medical Imaging BV, Maastricht, The Netherlands). This software automatically draws a centreline across the aortic lumen. After manual adjustment of the centreline, the software displays the short-axis view of the aortic root. Manual planimetry was used to trace the leading edges of the luminal and outer wall boundaries at 1 mm intervals of cross-sectional images. The plaque area was defined as the difference in area occupied by lumen area and vessel area. The entire aorta was divided into four segments: 1) the ascending aorta between the sinotubular junction and the origin of the brachiocephalic artery, 2) the aortic arch between the brachiocephalic artery and the left subclavian artery, 3) the descending aorta between the left subclavian artery and the diaphragm, and 4) the abdominal aorta below the diaphragm and above the bifurcation of the common iliac artery (Figure 1).
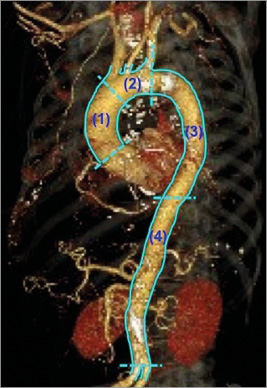
Figure 1. Analysed segments of aorta. The aorta was divided into four segments: (1) the ascending aorta, (2) the aortic arch, (3) the descending aorta, and (4) the abdominal aorta.
In each segment, total atheroma volume (TAV) was calculated by summation of the plaque area and subsequently normalised to account for difference in segment length between subjects (Figure 2):
![]()
Vessel and lumen volumes were calculated in a similar fashion.
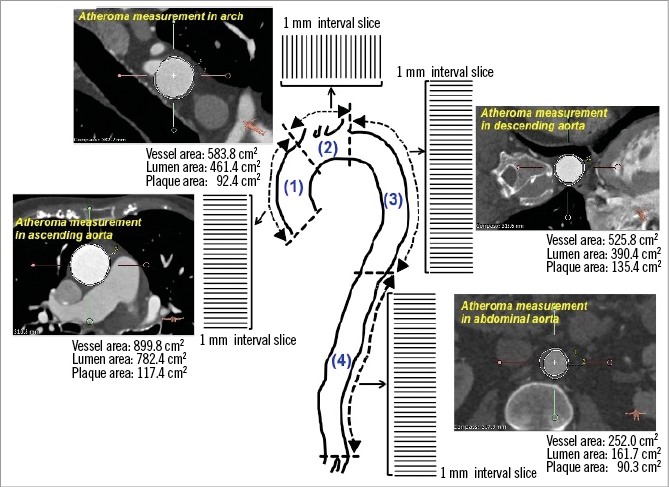
Figure 2. Volumetric analysis of aorta plaque burden. Lumen and outer wall boundaries were traced in every cross-sectional image at 1 mm intervals.
Calcium volume at the aortic valve complex and each cusp was also measured as previously published12. Since the average measurement of Hounsfield units in the lumen of the aorta was 500±100, a threshold of ≥700 Hounsfield units (=averaged value+2SD) was used to detect areas of calcium in the aortic valve complex.
ACUTE CVE
A neurologist was prospectively involved to identify CVE after TAVI, and to conduct cerebral CT scanning and appropriate therapeutic management. Acute CVE were defined as transient ischaemic attack or stroke within 24 hours after TAVI5,13. Transient ischaemic attack was defined as an acute focal neurological deficit lasting <24 hours without any evidence of cerebral infarction on imaging. Stroke was defined as an acute focal neurological deficit lasting >24 hours and/or with evidence of cerebral infarction on imaging. Stroke was classified as major stroke or minor stroke according to the modified Rankin Scale (major stroke: modified Rankin Scale score ≥2 at 30 days, minor stroke: modified Rankin Scale score <2 at 30 days).
STATISTICAL ANALYSIS
Continuous variables are expressed as mean±SD or median, and categorical variables as percentages. The chi-square test was used to test for differences in categorical variables between groups and continuous data were compared using unpaired t-tests, or Mann-Whitney log-rank tests when the variable was not normally distributed. Variables with a value of p<0.10 in the univariate logistic regression analysis were entered into a multivariate analysis to determine the independent predictors of acute CVE after TAVI. Receiver-operating characteristic analyses, and calculations of sensitivity and specificity were performed to analyse the predictive ability of TAV for acute CVE. The best cut-off value of TAVascending and TAVarch was determined by selecting the value which maximised the sum of sensitivity and specificity. A value of p<0.05 was considered significant. All statistical analyses were performed using JMP software, version 11.0.1 (SAS Institute, Cary, NC, USA).
Results
CLINICAL DEMOGRAPHICS
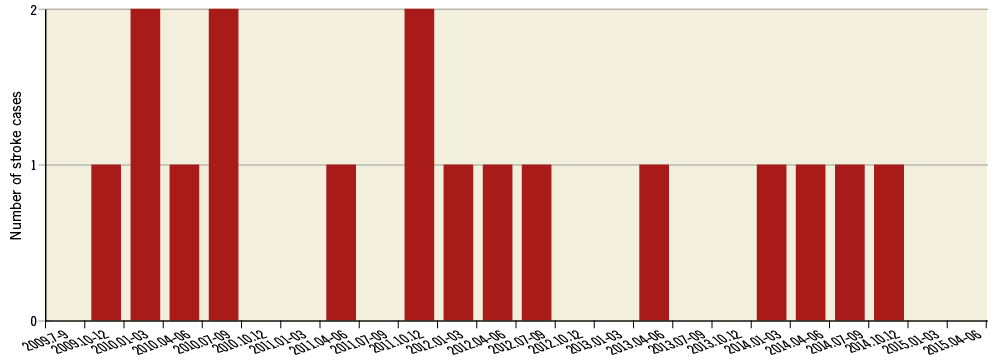
Sixteen patients had an acute CVE after TAVI (10 major and six minor stroke). Clinical characteristics are summarised in Table 1. Patients having acute CVE were more likely to have a history of cerebrovascular disease (p=0.002). There were no significant differences in other clinical demographics. The distribution and the detailed characteristics of CVE are summarised in Online Figure 1 and Online Table 1, respectively.
TAVI procedural data are summarised in Table 2. The femoral access was used in the majority of patients with and without acute CVE (p=0.20). The size and the type of prosthesis were similar between the two groups (p=0.61 and 0.58, respectively). There were no significant differences in stroke rate among four different valves (CoreValve 4.9% [6/122] vs. SAPIEN XT 6.8% [6/88] vs. Lotus 8.3% [3/36] vs. Portico 3.1% [1/32], p=0.39) and in self-expanding (CoreValve, Lotus and Portico) vs. balloon-expandable valves (SAPIEN XT) (5.2 vs. 5.6%, p=0.80), and Lotus valve vs. other valves (4.6 vs. 4.1%, p=0.77).
VOLUMETRIC MSCT ANALYSIS
Table 3 summarises MSCT measures in the two groups. On volumetric plaque analysis, greater TAVascending and TAVarch were observed in patients with acute CVE (12.8±3.5 vs. 7.0±2.1 cm3, p<0.001, 3.1±1.6 vs. 1.2±0.2 cm3, p<0.001), whereas there were no significant differences in TAV within the descending and abdominal aorta (29.5±11.8 vs. 29.3±11.0 cm3, p=0.75; 10.2±2.5 vs. 9.8±2.4 cm3, p=0.60). Vessel and lumen volumes were comparable in each segment between the two groups.
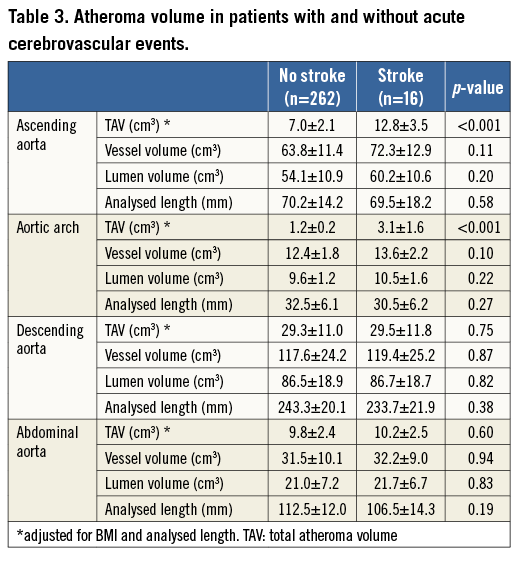
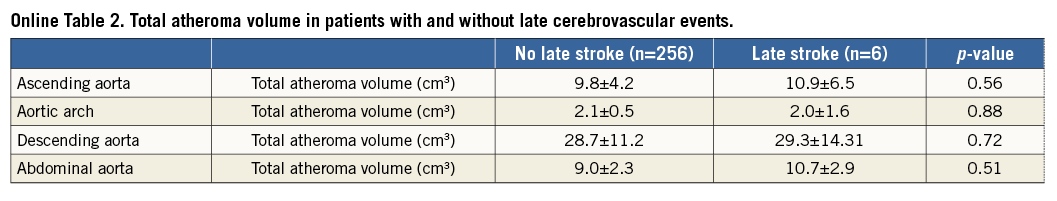
Of patients who did not experience acute CVE, late CVE (>24 hours after TAVI) were observed in six cases (6/262 [2.2%]). There were no significant differences in TAV between patients with and without late CVE (Online Table 2).
The calcification volume within the aortic valve complex and each cusp was also comparable (26.5±5.1 vs. 24.2±4.7 cm3, p=0.88; right cusp: 3.5±3.0 vs. 2.2±1.7 cm3, p=0.91, left cusp: 14.8±6.2 vs. 14.0±5.1 cm3, p=0.98, non-coronary cusp: 6.7±4.9 vs. 5.9±3.3 cm3, p=0.94).
OBSERVER VARIABILITY
Analysis of intra-observer variability was determined in 25,221 images acquired from 50 patients. The mean (±SD) differences were negligible for vessel wall (–0.10 cm2 [0.53 cm2]) and lumen areas (–0.04 cm2 [0.35 cm2]). Linear regression analysis showed close correlations between the original analysis and re-analysis (r=0.99 for vessel area, r=0.99 for lumen area). For inter-observer variability determination, the mean (±SD) differences were negligible for vessel wall (–0.18 cm2 [0.60 cm2]) and lumen areas (–0.14 cm2 [0.48 cm2]) in 25,221 images of 50 patients. Close correlations between the original analysis and subsequent analysis (r=0.97 for vessel area, r=0.97 for lumen area) were similarly observed.
PREDICTORS OF ACUTE CVE AFTER TAVI
Factors associated with acute CVE are shown in Table 4. A history of prior cerebrovascular disease (p=0.01), TAVascending (p=0.008) and TAVarch (p=0.009) were independent predictors of acute CVE. By receiver-operating curve analysis, the optimal cut-off value of TAVascending and TAVarch for predicting an acute CVE was 10.3 cm3 and 2.9 cm3, respectively (Figure 3).
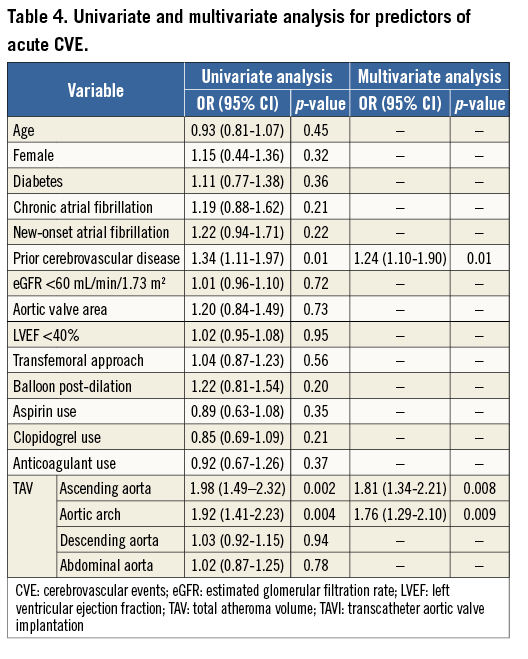
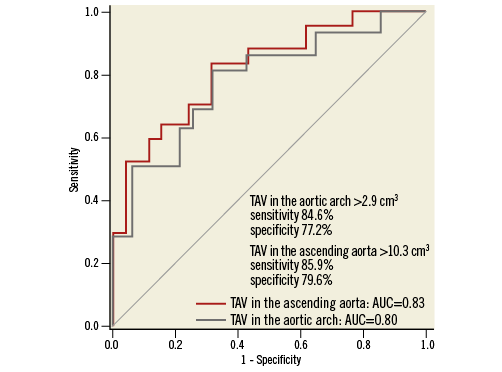
Figure 3. Receiver-operating characteristic analysis of TAVascending and TAVarch for predicting acute CVE after TAVI. CVE: cerebrovascular events; TAVarch: total atheroma volume in the aortic arch; TAVascending: total atheroma volume in the ascending aorta; TAVI: transcatheter aortic valve implantation
The incidence of acute CVE was compared in patients stratified into three groups according to the number of predictive features, including a history of cerebrovascular disease, TAVascending >10.3 cm3 and TAVarch >2.9 cm3 (Figure 4). While the incidence of acute CVE was 2.0% (4/196) in patients who had zero or one predictive feature, it was 6.7% (4/60) if patients had two predictive features. Moreover, patients having all three predictive features exhibited the highest incidence of acute CVE (36.4% [8/22], p<0.0001).
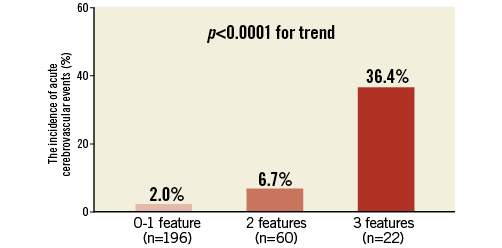
Figure 4. The incidence of acute CVE in patients having predictive features. The incidence of acute CVE was shown according to the number of predictive features: a history of cerebrovascular disease, TAVascending >10.2 cm3 and TAVarch >2.8 cm3. CVE: cerebrovascular events; TAVarch: total atheroma volume in the aortic arch; TAVascending: total atheroma volume in the ascending aorta
CLINICAL CHARACTERISTICS ASSOCIATED WITH TAVascending AND TAVarch
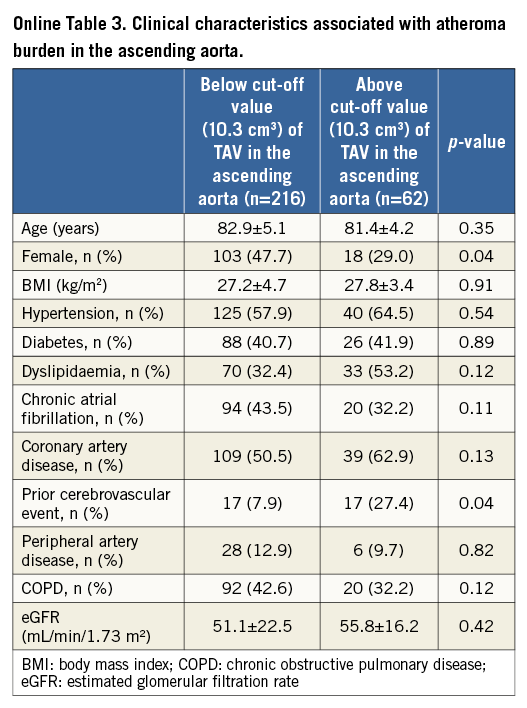
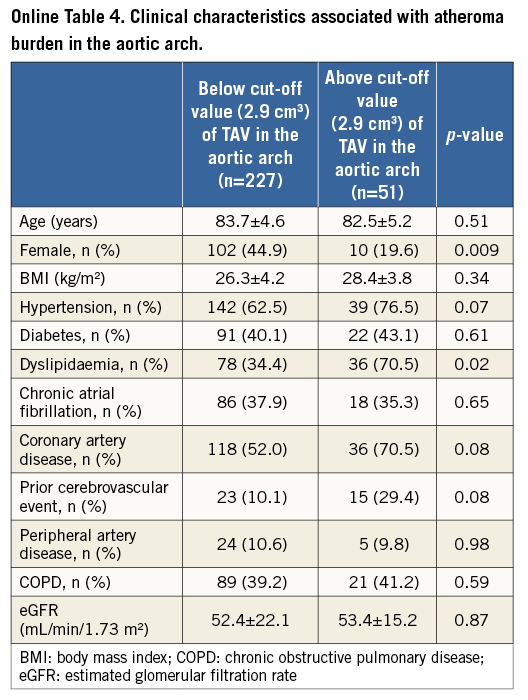
Further analysis was conducted to investigate clinical demographics associated with greater TAVascending and TAVarch (Online Table 3, Online Table 4). Patients with TAVascending above 10.3 cm3 were less likely to be female (p=0.04) and more likely to have a history of cerebrovascular disease (p=0.04). There was a lower proportion of females (p=0.009) and a higher prevalence of dyslipidaemia (p=0.04) in patients having TAVarch above 2.9 cm3.
Discussion
Dislodgement of debris from aorta atheroma burden during the passage of large-bore valve delivery catheters has been considered as a possible mechanism for acute CVE after TAVI. Our volumetric MSCT analysis showed that the occurrence of acute CVE was predicted by greater TAVascending and TAVarch. This finding is consistent with previous reports from transoesophageal echocardiography, showing a higher incidence of embolism after cardiac catheterisation in patients with evidence of aorta atherosclerosis14,15. Moreover, this incidence was higher in patients receiving intra-aortic balloon pump placement, which required the use of a larger size of catheter14. These observations support our hypothesis that patients receiving TAVI are at an increased risk of acute CVE if they have more TAVascending and TAVarch. As detached debris from the aortic wall between the ascending aorta and the aortic arch can travel to the brain through the brachiocephalic and left common carotid arteries, our MSCT findings may suggest an important distribution of aorta atherosclerosis associated with acute CVE after TAVI.
To date, there has been no study which has conducted volumetric MSCT analysis of aorta atherosclerosis. We sought to acquire cross-sectional images of the whole aorta to measure atheroma volume, as previously employed with intravascular ultrasound imaging of coronary atherosclerosis16. Inter- and intra-observer variability indicates that the current MSCT measurement approach is highly reproducible. A recent large cohort study reported balloon redilation and valve embolisation as independent predictors of acute CVE after TAVI5. However, it is hard to predict these procedural situations prior to TAVI. Therefore, evaluating atheroma burden might be a useful preoperative risk estimation of stroke after the procedure.
A history of cerebrovascular disease was identified as an independent predictor of acute CVE in the current study and in another recent article6. Patients having a prior cerebrovascular disease may harbour fragile atheroma in carotid arteries, potentially contributing to periprocedural stroke after TAVI. We could not delineate characteristics of carotid atherosclerosis as the number of patients who had baseline carotid imaging was limited.
A recent clinical trial has demonstrated the ability of cerebral protection devices to reduce ischaemic brain lesions and neurologic deficits after the procedure17. While this finding suggests the potential benefit of cerebral embolic protection devices, the appropriate indications for the use of this device have not yet been established. Our findings might suggest the ability of preoperative MSCT imaging of aorta atherosclerosis to identify patients who warrant cerebral embolic protection device use during TAVI.
The acute CVE rate in our study was higher compared to other recent studies4,5. One possibility is the learning curve effect. Twelve acute CVE were observed from 2009 to 2012, whereas there were four acute CVE from 2013 to 2015 (Online Figure 1). Less experience in procedure and post-procedural management, and the use of a catheter with a larger size might have contributed to a higher incidence of acute CVE in the early observational period.
Limitations
A number of caveats should be noted. The possibility of selection bias cannot be excluded, although consecutive subjects were included in the current analysis. The current study did not conduct MRI scans. This might have underestimated the incidence of CVE after the procedure. Quantification of atheroma burden by MSCT is affected by calcium blooming artefacts, potentially leading to the overestimation of calcified plaques. Plaque mobility and complexity and its composition may affect CVE, but its assessment is limited with MSCT. The small study population makes the analysis underpowered and a beta error cannot be ruled out with certainty. The ability of multivariate analysis to elucidate predictors with only 16 acute CVE is limited. The relationship of TAV with acute CVE should be further investigated in future studies with larger sample populations.
Conclusion
In summary, MSCT-derived aorta atheroma volume predicted acute CVE after TAVI in patients with severe symptomatic aortic valve stenosis. Our findings may underscore preoperative assessment of aorta atherosclerosis for risk estimation of acute CVE. Furthermore, this may have implications for the use of cerebral embolic protection devices in patients undergoing TAVI.
| Impact on daily practice CVE after TAVI are associated with morbidity and mortality. The interventional procedure is considered to induce embolism of atheromatous debris from the aortic wall. Volumetric MSCT analysis elucidated the association of TAVascending and TAVarch with acute CVE. Preoperative assessment of aorta atherosclerosis on MSCT might be useful to estimate the risk of acute CVE and identify patients requiring a cerebral embolic protection device during TAVI. |
Acknowledgements
The authors are grateful for the assistance of fellows and nurses at the Cardiovascular Investigational Unit and research nurses at the Cardiovascular Clinical Trials Unit of the Royal Adelaide Hospital.
Conflict of interest statement
Y. Kataoka has received research support from Cerenis. S. Nicholls has received speaking honoraria from AstraZeneca, Pfizer, Merck Schering-Plough and Takeda, consulting fees from AstraZeneca, Abbott, Atheronova, Esperion, Amgen, Novartis, Omthera, CSL Behring, Boehringer Ingelheim, Pfizer, Merck Schering-Plough, Takeda, Roche, NovoNordisk, LipoScience and Anthera, and research support from Anthera, AstraZeneca, Cerenis, Eli Lilly, InfraReDx, Roche, Resverlogix, Novartis, Amgen and LipoScience. S. Worthley has received research grants and honoraria from St. Jude Medical and Medtronic. The other authors have no conflicts of interest to declare.
Supplementary data
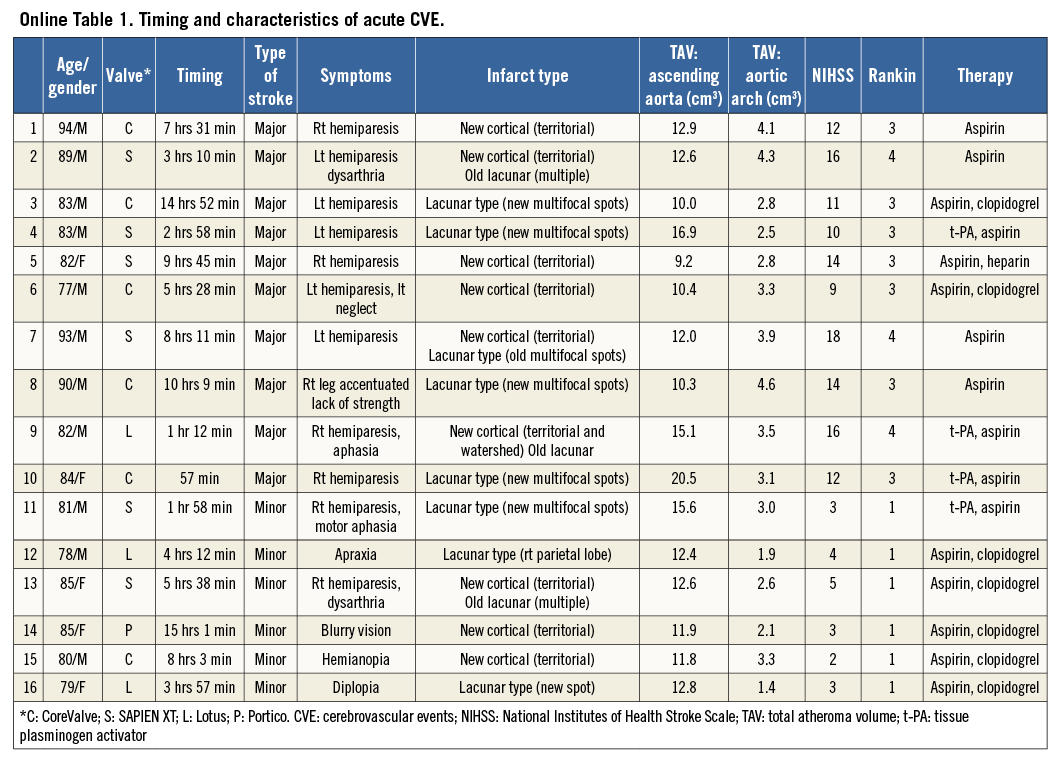
Online Figure 1. Distribution of acute CVE.

