
Abstract
Aims: The newly formed geometry between the native Valsalva and implanted transcatheter heart valve (THV) may induce local thrombogenicity. This study aimed to assess the incidence of and the clinical outcomes associated with Valsalva thrombus formation after transcatheter aortic valve implantation (TAVI).
Methods and results: We retrospectively evaluated the multidetector computed tomography (MDCT) data of 338 patients following transcatheter aortic valve implantation (TAVI) using a balloon-expandable THV. The Valsalva and leaflet thrombi were assessed by MDCT at the left coronary cusp (LCC), right coronary cusp (RCC), and non-coronary cusp (NCC). Combined endpoints such as death, stroke, and readmission for heart failure rates in patients with and without Valsalva and/or leaflet thrombus were examined at two years. The overall incidence of Valsalva and leaflet thrombi was 8.9% and 8.3%, respectively. Significant differences in the location of the Valsalva thrombus in the LCC, RCC, and NCC were noted (5.0%, 4.2%, 8.9%, respectively, p<0.001). The independent predictor for increased risk of Valsalva thrombus was high Valsalva area to implanted THV size ratio (odds ratio 11.8, 95% confidence interval [CI]: 1.67-83.0, p=0.013). Combined endpoints were similar in patients with and without Valsalva thrombus, Valsalva/leaflet thrombus, and leaflet thrombus (p>0.05 for all).
Conclusions: Valsalva thrombus was detected in 8.9% of patients following balloon-expandable THV implantation and was common in the LCC, but it did not increase the risk of adverse events after TAVI.
Introduction
With the development of devices and technical improvements, the indications for transcatheter aortic valve implantation (TAVI) for severe aortic stenosis (AS) have been expanding to patients considered to be in the intermediate surgical risk category1. To date, the existence of leaflet thrombus in patients who underwent TAVI is one of the important remaining issues when considering indications for TAVI2. Many recent studies have revealed the incidence, mechanism, and predictive factors of leaflet thrombus following TAVI3,4,5,6,7,8,9,10,11. The majority of patients with leaflet thrombus are free from clinical adverse events, and thus it is called “subclinical” leaflet thrombosis3,4,5,6,7,8. However, clinically relevant leaflet thrombus, although rare, should not be ignored10,11. Pivotal research has mentioned the probable increased risk of minor stroke events associated with leaflet thrombosis12. We also previously reported a case of huge Valsalva thrombus formation between the native Valsalva and the implanted transcatheter heart valve (THV) after TAVI13. Caution must be exercised not only concerning the existence of leaflet thrombus, but also for Valsalva thrombus in patients who have undergone TAVI. Up until now, there are no available data concerning the incidence and prognostic value of Valsalva thrombus following TAVI. This study, therefore, aimed to assess the existence of Valsalva thrombus and to clarify the clinical outcomes of Valsalva thrombus after TAVI using the data from 338 patients enrolled in a Japanese multicentre registry.
Methods
This study used data from an ongoing multicentre registry, the Optimized CathEter vAlvular iNtervention-transcatheter aortic valve implantation (OCEAN-TAVI) registry, involving 14 relatively high-volume centres in Japan14. This trial was registered with the University Hospital Medical Information Network (no.: UMIN000020423). Data for a total of 338 aortic stenosis (AS) patients were retrospectively extracted from the OCEAN-TAVI registry for the period from October 2013 to November 2016. All 338 patients had undergone four-dimensional multidetector computed tomography (MDCT) examinations after TAVI (median duration from TAVI to MDCT: 2 days, interquartile range [IQR]: 2 to 7 days). During the hospital stay and follow-up period, experienced echocardiographers in each centre calculated the conventional findings using transthoracic echocardiography (TTE). Information regarding the occurrence of severe adverse events and death was collected from the treating hospitals or by calling the patient’s family member(s).
Details of the TAVI procedure have been reported previously14. The total number of patients implanted with the SAPIEN XT and SAPIEN 3 (Edwards Lifesciences, Irvine, CA, USA) balloon-expandable prostheses was 229 and 109, respectively. The TAVI procedures were performed with patients under anticoagulant therapy with a bolus of unfractionated heparin administered to achieve an activated clotting time of >250 seconds, which was maintained throughout the procedures. Procedural complications and clinical outcomes, including the cause of death, were evaluated according to the Valve Academic Research Consortium-2 criteria15.
All 338 patients were scanned using four-dimensional enhanced MDCT with electrocardiography-gated reconstructions. MDCT data sets were previously mentioned in detail8. The Valsalva thrombus was defined as a low-density space without enhancement of contrast media between the native Valsalva and implanted THV13. The definition of leaflet thrombus was according to that described in our previous report8. Baseline characteristics, procedural parameters, and clinical outcomes were compared between the with thrombus (Valsalva or leaflet) and without thrombus groups. Representative images of the Valsalva thrombus and leaflet thrombus are presented in Figure 1. Locations of both Valsalva and leaflet thrombi were evaluated for each Valsalva position in the left coronary cusp (LCC), right coronary cusp (RCC), and non-coronary cusp (NCC). The Valsalva length, THV length, and Valsalva length to THV length ratio, Valsalva area, THV area, and Valsalva area to THV area ratio were calculated (Figure 2). The depth of each prosthesis implantation was measured as the maximum distance from the bottom of the stent to the native annulus9. The angle of the native commissure to bioprosthetic leaflet orientation was assessed in each coronary cusp. Anti-anatomical position was defined as rotation of the prosthetic valve commissure post on nearly both the coronary artery ostia and interatrial septum according to previous reports16.
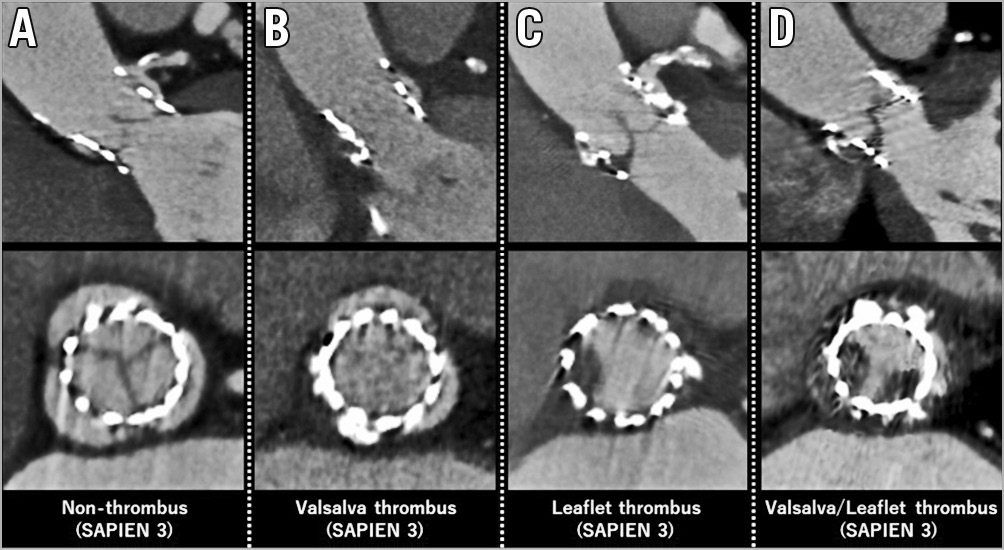
Figure 1. Representative thrombus images. A) No thrombus. B) Valsalva thrombus. C) Leaflet thrombus. D) Both Valsalva and leaflet thrombi.
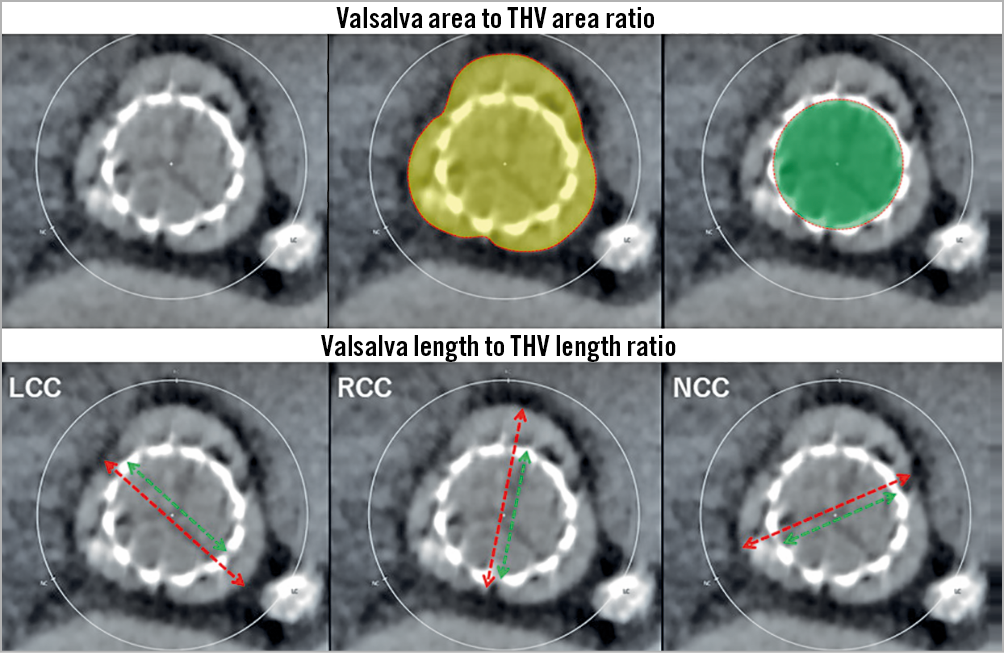
Figure 2. The Valsalva to transcatheter heart valve (THV) ratios in terms of area and length. Valsalva area to THV ratio was calculated by the yellow area divided by the green area (upper panels). Valsalva length to THV length ratio was calculated by the green distance divided by the red distance for each Valsalva position in the left coronary cusp (LCC), right coronary cusp (RCC), and non-coronary cusp (NCC) (lower panels).
STATISTICAL ANALYSIS
All statistical analyses were performed using SPSS Statistics, Version 22 (IBM Corp., Armonk, NY, USA). Continuous variables are expressed as mean±SD and as medians with IQR, depending on the distribution, while the categorical data are expressed as numbers and percentages of the total. Comparisons of the two groups were undertaken using chi-squared or Fisher’s exact tests for covariates expressed using mean and SDs, and the Mann-Whitney U test for continuous variables expressed as medians with IQR. The Kaplan-Meier method was used to estimate the combined endpoint of cumulative mortality, stroke and readmission for heart failure in the with and without Valsalva thrombus groups, as well as in the with and without leaflet thrombus groups. A univariate regression analysis predicted the odds ratio regarding the existence of Valsalva thrombus and leaflet thrombus in each variable. Thereafter, a multivariate analysis was performed using the baseline clinical characteristics and other variables with a univariate p-value <0.1 in order to examine independent associations of Valsalva thrombus and leaflet thrombus. All statistical tests were two-sided, and values with p<0.05 were considered statistically significant.
Results
Age, sex, body characteristics, comorbidities, blood examinations, and medical treatments were not different between the with Valsalva thrombus and without Valsalva thrombus groups (Table 1), as well as the with leaflet thrombus and without leaflet thrombus groups (Supplementary Table 1). The TTE parameters were similar in the two groups with respect to both Valsalva and leaflet thrombi. Procedural antiplatelet and anticoagulant therapy was not significantly different between the groups.
Preprocedural and post-procedural MDCT parameters are presented in the Valsalva groups (Table 2) and in the leaflet groups (Supplementary Table 2). The annulus size as measured by MDCT was not different between the groups. However, the sizes of the LCC, RCC, and NCC were larger in the Valsalva thrombus group than those in the without Valsalva thrombus group. These trends were also confirmed in the leaflet thrombus and without leaflet thrombus groups. The Valsalva length (except for LCC) and area to those of THV ratios were also larger in the Valsalva thrombus group than in the without Valsalva thrombus group (all p<0.05). Similar differences were observed in the with leaflet thrombus and without leaflet thrombus groups (all p<0.05). The angle of the native commissure to bioprosthetic leaflet orientation was approximately 30 degrees rotated, whereas the incidence of Valsalva and leaflet thrombus did not differ. The distributions of the Valsalva and leaflet thrombi were compared overall, and at each Valsalva location (Figure 3). The overall incidence of Valsalva thrombus was 8.9% (30/338). Leaflet thrombus was found in 28 of 338 patients (8.3%). Altogether, 28.6% of the Valsalva thrombi were partially located inside the leaflet (n=8/28). Conversely, 26.7% of the leaflet thrombi overlapped with a Valsalva thrombus (n=8/30). The prevalence of Valsalva thrombus in the LCC and RCC was lower compared with that in the NCC (5.0% [17/338] vs 4.1% [14/338] vs 8.9% [30/338], p<0.001). These trends were similarly found in the SAPIEN XT valve (p<0.001) and SAPIEN 3 valve (p<0.001). The incidences of Valsalva and leaflet thrombi were also compared with respect to the balloon-expandable valve sizes (Figure 4). Among the balloon-expandable valves (20 mm vs 23 mm vs 26 mm vs 29 mm), the incidence of Valsalva thrombus was 6.7% (1/15), 9.0% (19/212), 9.1% (9/99), and 8.3% (1/12), respectively (p=0.75), and that for leaflet thrombus was 6.7% (1/15), 7.1% (15/212), 7.1% (7/99), and 33.3% (4/12), respectively (p=0.27).
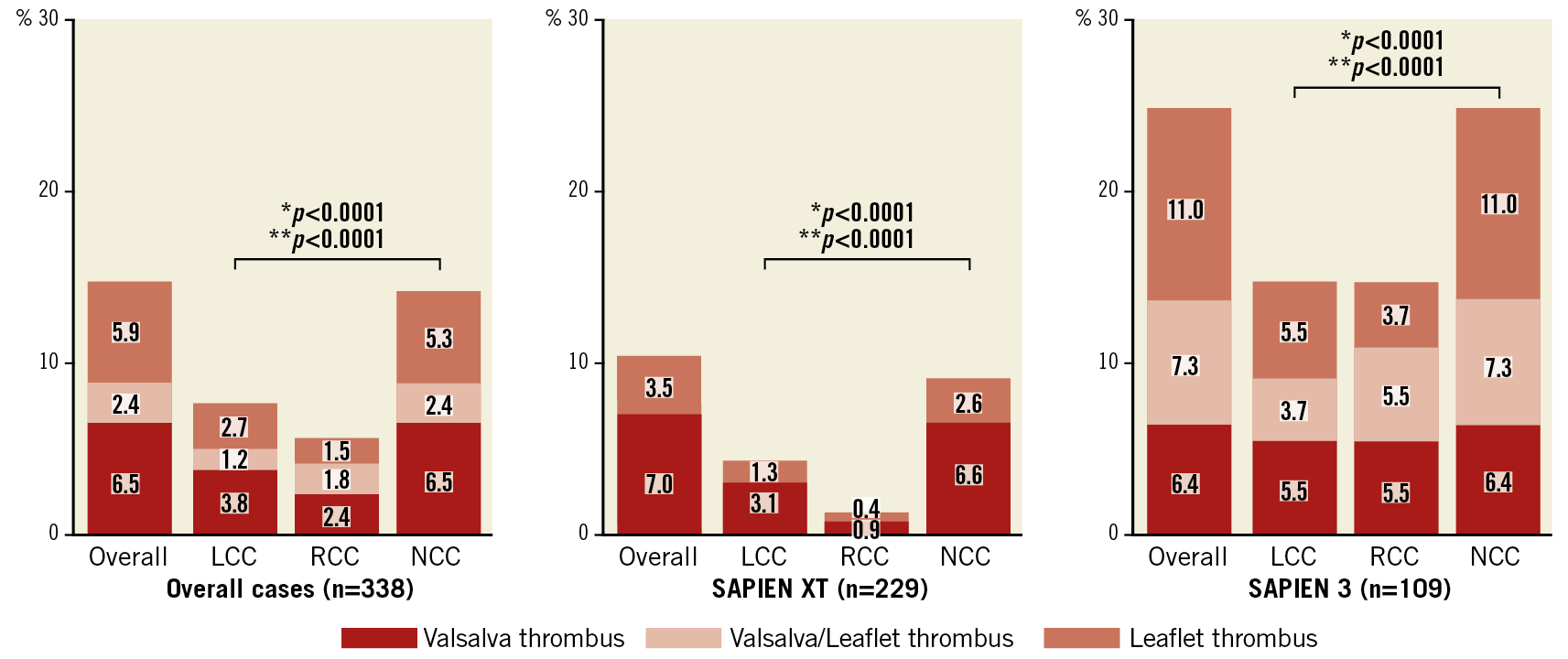
Figure 3. Distributions of Valsalva, Valsalva leaflet, and leaflet thrombi overall, and in SAPIEN XT valve and SAPIEN 3 valve groups. *p for the incidence of Valsalva thrombus. **p for the incidence of leaflet thrombus.
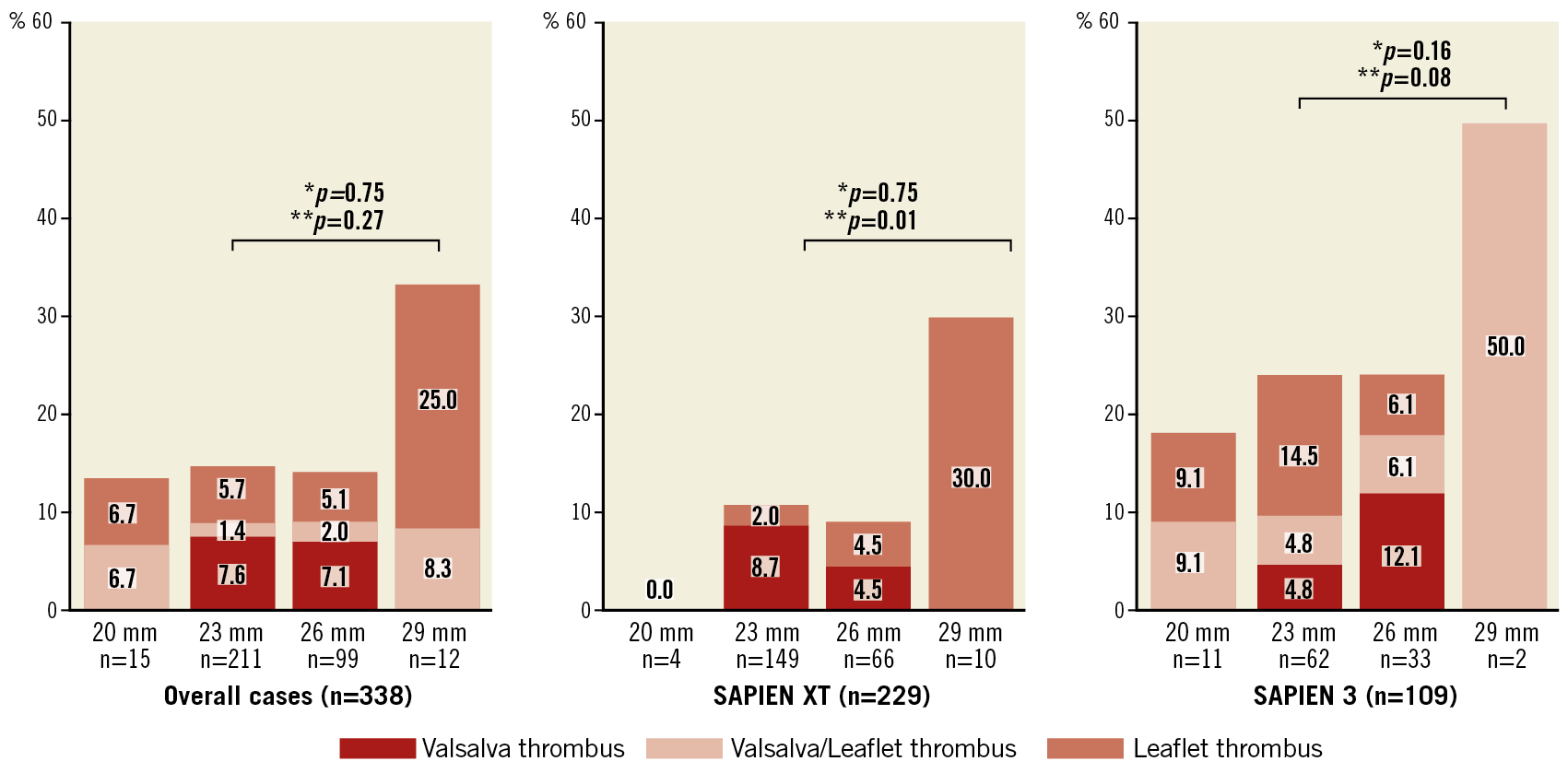
Figure 4. Comparison of incidences of Valsalva, Valsalva leaflet, and leaflet thrombi in overall, SAPIEN XT, and SAPIEN 3 valve sizes. *p for the incidence of Valsalva thrombus. **p for the incidence of leaflet thrombus.
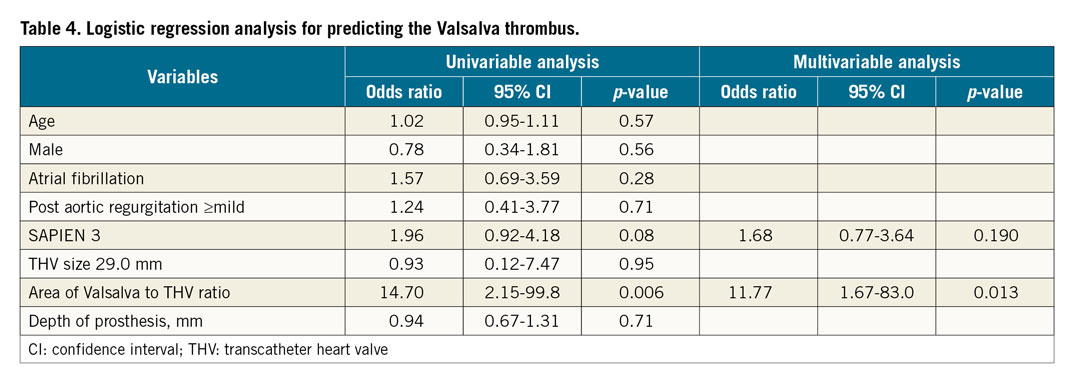
Post-procedural echocardiographic parameters and rates of procedural complications are described in the Valsalva groups (Table 3) and in the leaflet groups (Supplementary Table 3). Post-procedural mean pressure gradient was similar in the Valsalva thrombus and without Valsalva thrombus groups. No significant differences were observed between each group concerning the rates of in-hospital death and procedural complications. Clinical follow-up was performed up to two years after TAVI. Kaplan-Meier analysis showed no significant differences in the combined endpoint in patients in the with Valsalva thrombus and without Valsalva thrombus groups (Figure 5A) and in the with leaflet thrombus and without leaflet thrombus groups (Figure 5B). Combining all patients with Valsalva and/or leaflet thrombi (n=51), the incidence of the combined endpoint was similar between the patients with thrombus and without thrombus (Figure 5C). The logistic regression analysis of predictive factors for Valsalva thrombus and leaflet thrombus is shown in Table 4 and Supplementary Table 4, respectively. In the multivariate model, the area of Valsalva to THV ratio was an independent predictive factor of Valsalva thrombus. Low flow, severe prosthesis-patient mismatch, SAPIEN 3 valve, the area of Valsalva to THV ratio, and THV size 29 mm were independent predictive factors for leaflet thrombus.
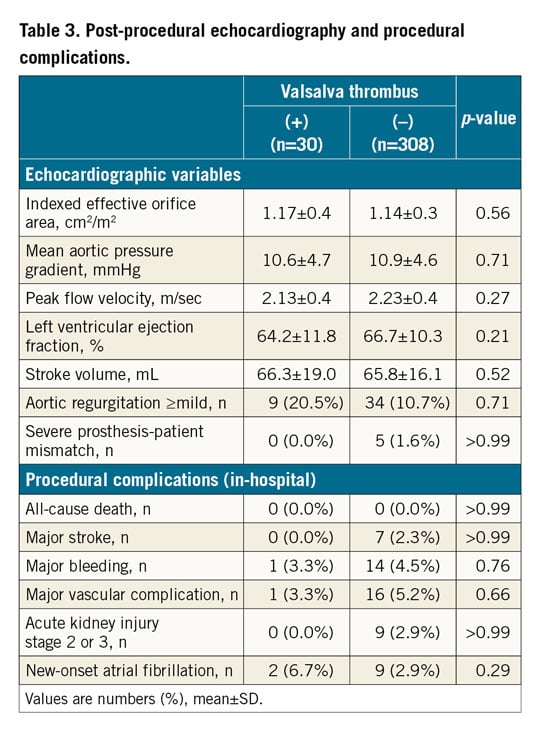
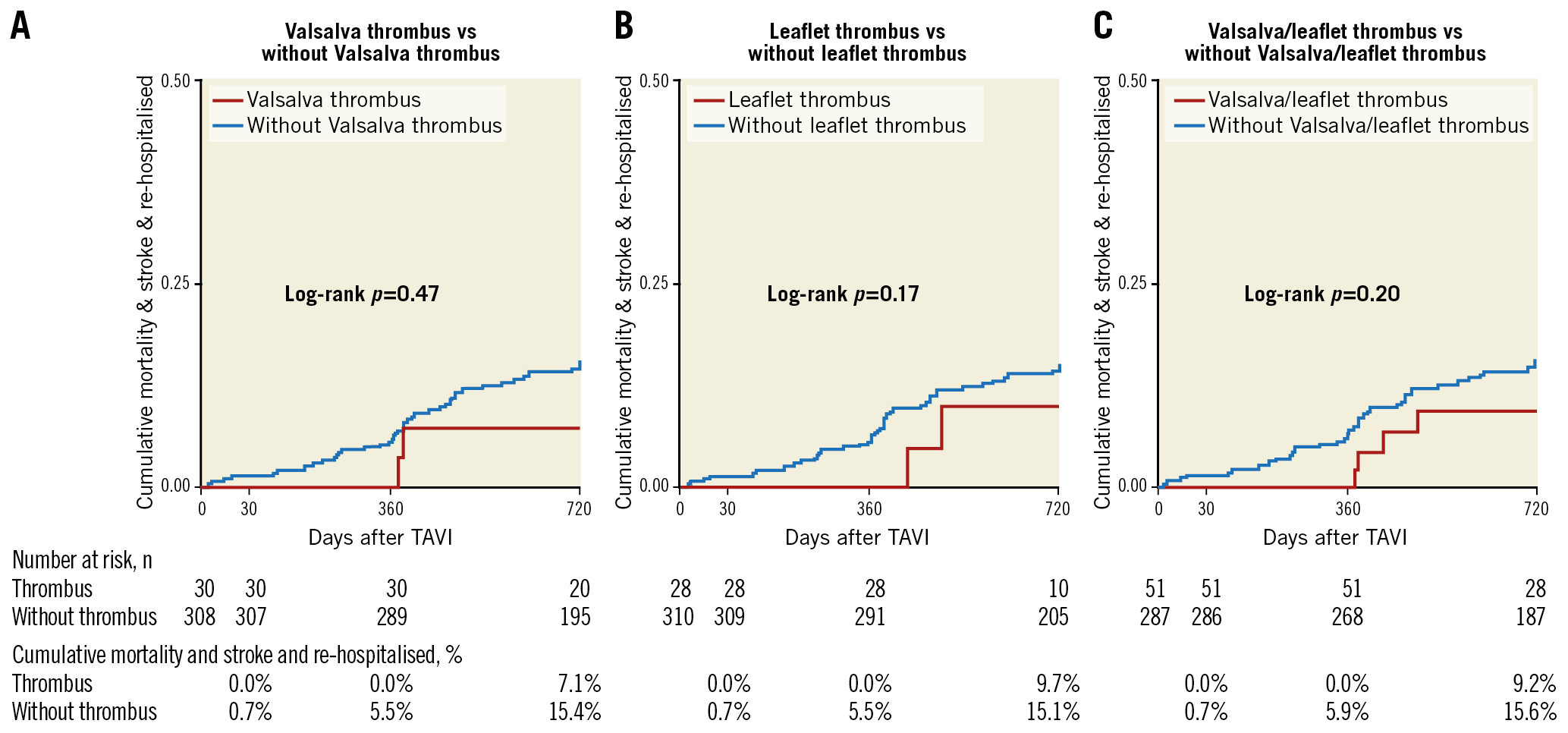
Figure 5. Cumulative mortality and stroke rates between thrombus and non-thrombus groups. Kaplan-Meier curves showing combined endpoint among the three groups (A-C).
Discussion
Altogether, the present study demonstrated new findings regarding Valsalva thrombus formation between the native Valsalva and implanted THV following TAVI. Although Valsalva thrombus formation was not significantly associated with all-cause death and stroke events, clinically silent Valsalva thrombus was found in 8.9% of the patients (30/338) who had undergone TAVI. In our study, the timing of MDCT examination was a median of three days after TAVI. The interval from the TAVI procedure to MDCT varied significantly among previous studies, ranging from several days to months (3 to 12). Although the reason why valve thrombosis was recognised in such an early phase was unclear, early leaflet thrombus formation was confirmed by our data and our previous report. In addition, Valsalva thrombus formation was identified during the same early phase after TAVI. Thus, the potential risk of early thrombosis might be affirmed in both the Valsalva and leaflet in patients who underwent TAVI.
Numerus considerations have been discussed about the causation of leaflet thrombus: these involve speculation regarding the implanted large valve size, balloon-expandable valve type, stent frame geometry, technical aspects, conditions of medical therapy, and fluid mechanics among others6,7,8,9,10. The incidence of leaflet thrombus was also significantly higher with SAPIEN 3 valves than with SAPIEN XT valves. However, the sample size was limited in our study and there are arguments both for and against the incidence of leaflet thrombus in the valve differences5,6,12. Thus, this topic needs to be investigated in further studies.
The newly formed geometry of the implanted THV affects fluid mechanics negatively in the aorto-valve complex and causes leaflet thrombus formation after TAVI9. Fluid mechanics is an important factor when considering the mechanism of THV leaflet thrombosis. An in vitro study also proved that the space between the implanted THV and native Valsalva triggered a non-physiological and downstream flow17,18. Moreover, the Valsalva space was thought to be a potential risk factor for thrombosis after TAVI13. In fact, the area of Valsalva to THV ratios, in terms of both length and area, were significantly larger in the Valsalva thrombus group than in the without Valsalva thrombus group as well as in the leaflet thrombus group. The stent frame design creates more space between the native Valsalva and implanted THV, and thus, as expected, the incidence of Valsalva and leaflet thrombus was higher in the large Valsalva space. The Valsalva thrombus was also located significantly more often in the NCC area. This finding can be explained by the absence of coronary flow in the NCC area in contrast to the other Valsalva cusps. Several factors are related to the increase in local thrombogenicity inside the Valsalva space. As a result, the overall incidence of THV thrombus formation inside the aorto-valve complex might be higher than expected because earlier studies only focused on the existence of leaflet thrombus.
Both Valsalva and leaflet thrombi identified by MDCT were clinically silent. This was classified as subclinical thrombosis in this study, supporting the findings of many past studies3,4,5,6,7,8. The clinical question under debate regards medical intervention using antithrombotic therapy for preventing leaflet thrombus. Anticoagulant therapy is considered to be effective for reducing leaflet thrombosis3,6,11,12,19. A recent large-scale multicentre data analysis reported that the lack of anticoagulation therapy post TAVI was associated with significant increments in transvalvular gradient and a greater risk of valve haemodynamic deterioration20. However, contradictory data exist suggesting that the natural history of untreated leaflet thrombus is not related to an increase in the pressure gradient after TAVI4,5,8,21. When considering medical intervention for valve thrombosis, it is important to assess the clinical relevance of valve thrombus that is associated with valve deterioration, stroke, and heart failure. Although rare, significant elevation of the transvalvular pressure gradient was related to the massive leaflet thrombus detected by MDCT11. A study revealed that valve thrombosis was a possible cause of thromboembolic stroke events22. In such kinds of high-risk subsets, additional anticoagulant therapy might be useful to treat the adverse clinical events. However, our data demonstrated no significant increased risk of the combined endpoint in patients with leaflet and Valsalva thrombi. The routine use of anticoagulation therapy for its preventive effect against THV thrombus formation should be carefully decided on the basis of the background of each individual patient, especially when considering bleeding risk in the very elderly TAVI patient cohort. Moreover, large-scale data will be required to define an optimal medical therapy after TAVI.
Limitations
Several limitations of this study should be discussed. First, this was a retrospective study without definitive inclusion criteria for MDCT examinations. Patients with renal dysfunction were excluded, thus selection bias in patient enrolment was inevitable. Second, it is difficult to prove the direct relationship between the Valsalva/leaflet thrombus and minor stroke events because the MDCT assessments for the existence of THV thrombosis did not take place when clinical events occurred. In addition, this registry did not have core laboratory centres and neurological specialists who could evaluate the stroke rates properly. Therefore, we should not overstate our conclusions concerning the lack of clinical association between THV thrombus and minor stroke events. Third, the long-term follow-up data of echocardiography were not validated in all patients. Therefore, the clinical influence of Valsalva and/or leaflet thrombus on the occurrence of structural valve deterioration could not be evaluated in this study.
Conclusions
Altogether, Valsalva thrombus was found in 8.9% of patients following TAVR and was especially more common in the LCC. However, both Valsalva and/or leaflet THV thrombi were not related to an increased risk of adverse events. Further clinical investigations will be required to assess the clinical implications of THV thrombosis.
|
Impact on daily practice Valsalva thrombus was found in 8.9% of patients following TAVR and was especially more prevalent in the LCC and the large Valsalva space to the implanted THV. Although Valsalva thrombus was subclinical, the optimal management for patients with these TAVR-specific complications needs to be clarified. |
Acknowledgements
The authors wish to thank all the OCEAN-TAVI investigators.
Funding
The OCEAN-TAVI registry is supported by Edwards Lifesciences and Daiichi Sankyo.
Conflict of interest statement
M. Yamamoto, T. Naganuma, K. Mizutani, and Y. Watanabe are clinical proctors for Edwards Lifesciences and Medtronic. M. Araki, A. Higashimori, and K. Hayashida are clinical proctors of Edwards Lifesciences. The other authors have no conflicts of interest to declare.

