Abstract
Symptomatic transcatheter heart valve (THV) thrombosis is noted in up to 1% of patients after transcatheter aortic valve replacement. Recently, hypoattenuated leaflet thickening and reduced leaflet motion of bioprosthetic aortic valves associated with normal transvalvular gradients (and possibly related to subclinical leaflet thrombosis) have been reported. While transthoracic echocardiography is a useful initial screening imaging modality for the detection of symptomatic THV thrombosis associated with elevated transvalvular gradients, it has limited utility in the detection of subclinical THV thrombosis. In the present article, we describe the incidence, clinical presentation and strategies for the prevention and treatment of THV thrombosis, as well as the role of imaging modalities in the diagnosis and management of this phenomenon.
Introduction
Symptomatic transcatheter heart valve (THV) thrombosis is noted in up to 1% of patients after transcatheter aortic valve replacement (TAVR)1. Recently, hypoattenuated leaflet thickening (HALT) and reduced leaflet motion (RELM) of bioprosthetic aortic valves associated with normal transvalvular gradients (possibly related to subclinical leaflet thrombosis) have been reported as affecting both transcatheter and surgical aortic valves2-4. While symptomatic THV thrombosis is rare, subclinical leaflet thrombosis occurs relatively frequently. The purpose of this manuscript is to describe the incidence, clinical presentation and strategies for the prevention and treatment of THV thrombosis (clinical and subclinical), as well as the role of imaging modalities in the diagnosis and management of this phenomenon.
Symptomatic THV thrombosis
The first case of symptomatic thrombotic obstruction of the Edwards SAPIEN valve (Edwards Lifesciences, Irvine, CA, USA) was reported by Trepels et al5. Lancellotti et al reported the first case of symptomatic thrombotic occlusion of the Medtronic CoreValve® (Medtronic, Minneapolis, MN, USA)6. The majority of the reported cases of THV thrombosis have been in symptomatic patients, the clinical presentation ranging from cardiopulmonary arrest7, recurrence of symptoms on follow-up6,8,9 to non-ST-elevation myocardial infarction8. Thrombotic THV have been treated with anticoagulation with successful resolution of thrombus8-10 or surgical aortic valve replacement (SAVR)5,6. Latib et al reported the frequency, clinical presentation and management of symptomatic THV thrombosis from a multicentre registry of 26 cases of symptomatic THV thrombosis1. The overall incidence of symptomatic THV thrombosis was 0.61%. All patients in the registry were symptomatic (most commonly presenting with worsening shortness of breath), and elevated transaortic gradients were detected in 92.3% (24/26) of the patients. THV thrombosis presented as thickened leaflets or thrombotic apposition of leaflets in 76.9% and thrombotic mass on the leaflets in 23.1%. Twenty-three out of 26 patients (88.4%) were successfully treated with anticoagulation; two patients underwent a transcatheter valve-in-valve procedure and one patient underwent SAVR. Figure 1 depicts an example of a SAPIEN 3 (Edwards Lifesciences) valve thrombosis associated with elevated and worsening gradients, successfully treated with anticoagulation. Figure 2 presents an example of a THV stenosis with elevated aortic valve gradients treated as a degenerative THV with THV-in-THV procedure, complicated by ostial right coronary artery obstruction. Post hoc, the appearance of the valve leaflets on transoesophageal echocardiography (TEE) and computed tomography (CT) was suggestive of thrombus, with RCA obstruction caused by thrombus protruding into the RCA ostium.
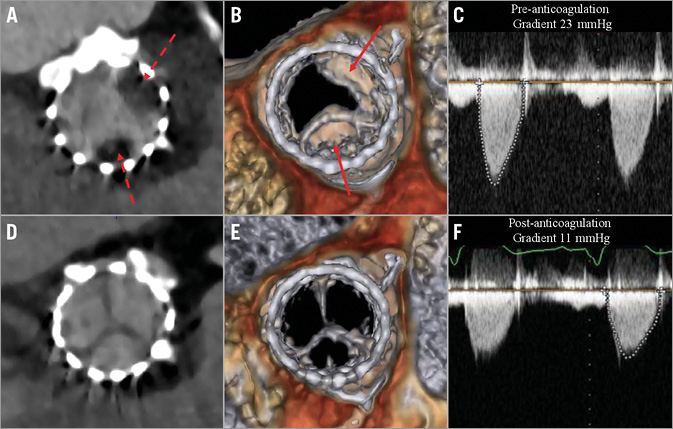
Figure 1. Resolution of reduced leaflet motion of SAPIEN 3 valve with anticoagulation. Hypoattenuated leaflet thickening (HALT) on short-axis two-dimensional CT (A) and reduced leaflet motion (RELM) on volume-rendered imaging (B) associated with elevated aortic valve gradient (C). Following three months of anticoagulation with warfarin, repeat CT revealed resolution of HALT (D) and RELM (E) with restoration of normal aortic valve gradient (F).
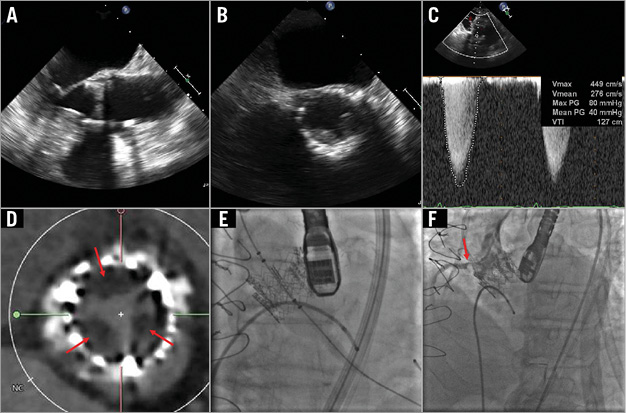
Figure 2. Case example of symptomatic transcatheter heart valve (THV) thrombosis. Left ventricular outflow tract (A) and short-axis (B) TEE views showing thickened THV leaflets with reduced mobility during systole. C) Elevated transaortic gradient across the THV consistent with clinically significant obstruction. D) CT appearance of hypoattenuating opacities on the THV leaflets suggestive of thrombus. E) THV-in-THV implantation for symptomatic THV failure. F) Ostial right coronary artery (RCA) obstruction caused by protrusion of the thrombus into the RCA ostium.
Subclinical THV thrombosis
PREVALENCE OF HALT/RELM
Three studies have reported the prevalence of this finding in bioprosthetic aortic valves. Leetmaa et al performed computed tomograms (CT) in 140 patients with SAPIEN XT valves (Edwards Lifesciences) at one to three months post TAVR4. THV thrombosis (defined as HALT) was present in five patients (4%), four of these patients being asymptomatic with no echocardiographic evidence of significantly elevated gradients. Pache et al performed contrast CT in 156 patients undergoing TAVR with the SAPIEN 3 valve at a median of five days post TAVR3. HALT was noted in 16 (10.3%) patients.
Makkar et al reported their findings from 55 patients enrolled in the ongoing PORTICO trial (1:1 randomisation to the Portico™ valve [St. Jude Medical, St. Paul, MN, USA] versus commercially available THVs), and from 132 patients undergoing TAVR or surgical aortic valve replacement (SAVR) enrolled in two single-centre registries (The Assessment of Transcatheter and Surgical Aortic Bioprosthetic Valve Thrombosis and Its Treatment with Anticoagulation [RESOLVE] registry, and The Subclinical Aortic Valve Bioprosthesis Thrombosis Assessed with Four-Dimensional Computed Tomography [SAVORY] registry)2. While Leetma et al and Pache et al evaluated THVs for HALT, this series further evaluated the presence of RELM using 3D volume-rendered (VR) imaging. RELM was noted in 39 of 187 (20.9%) patients and in multiple THV types, including the Portico valve, Edwards valves (Edwards SAPIEN, SAPIEN XT and SAPIEN 3), Medtronic CoreValve and the Lotus™ valve (Boston Scientific, Marlborough, MA, USA) (Figure 3).
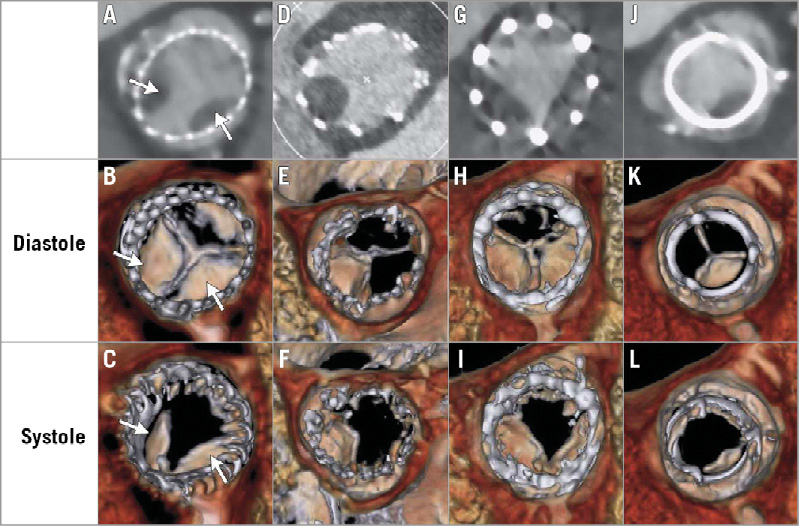
Figure 3. Evidence of reduced leaflet motion in multiple prosthesis types. Hypoattenuating opacities on two-dimensional (maximum intensity projection of greyscale image) and volume-rendered CT (colour images) for multiple prosthesis types, including the CoreValve (panels A-C, arrows), Portico (panels D-F), SAPIEN XT (panels G-I), and Carpentier-Edwards PERIMOUNT surgical valve (Edwards Lifesciences) (panels J-L) during diastole and systole. Normal leaflets are visible only on volume-rendered CT in diastole, at their line of coaptation in axial images. Leaflets with reduced motion are visible as wedge-shaped or semilunar opacities in both systole and diastole. Adapted from Figure 2, N Engl J Med. 2015;373:2015-24.
ROLE OF ECHOCARDIOGRAPHY IN THE DETECTION OF HALT/RELM
The normal function of a bioprosthetic valve is traditionally defined by normal leaflet mobility without increased AV gradients or central aortic regurgitation. Although TTE may exclude stenosis or incompetence, it has limited utility in the detection of HALT/RELM due to inadequate visualisation within the valve stent frame and the presence of normal aortic valve gradients. In the series reported by Leetmaa et al, four out of five patients were asymptomatic, with no evidence of significantly elevated gradients4. Likewise, Makkar et al did not notice any difference in the mean aortic valve gradients between patients with or without reduced leaflet motion2. Although Pache et al reported significantly higher mean gradients in patients with HALT compared to those without (11.6±3.4 vs. 14.9±5.3 mmHg, p=0.026), a mean gradient of 14.9±5.3 mmHg is still considered “normal” for THVs; thus, the difference does not seem to be clinically significant3. The finding of RELM was confirmed with TEE in all cases in the reported series; however, the utility of TEE is limited due to its being an invasive imaging modality. Although TEE was used to confirm the findings following a CT scan, its utility as a standalone screening modality has not been established.
IMPACT OF ANTICOAGULATION ON HALT/RELM
In the series by Leetmaa et al, there were no cases of HALT noted in anticoagulated patients (0/5 cases of THV thrombosis). Makkar et al reported similar findings, with no cases of RELM noted in patients who were therapeutically anticoagulated with warfarin compared to those on dual antiplatelet therapy (0% and 55%, respectively, p=0.01 in the PORTICO clinical trial; and 0% and 29%, respectively, p=0.04 in the pooled RESOLVE and SAVORY registries). In contrast to these findings, Pache et al did not observe significant interaction between anticoagulation and THV thrombosis. However, the median time to CT was five days post TAVR in this series, and it is likely that levels of anticoagulation were subtherapeutic at the time of the CT scan. Initiation of anticoagulation following detection of HALT/RELM resulted in resolution of the imaging findings and restoration of normal leaflet motion in all patients2-4.
IMPACT ON CLINICAL OUTCOMES
There is a paucity of data on the impact of HALT/reduced leaflet motion on clinical outcomes. Leetmaa et al and Pache et al provided limited clinical follow-up, with no strokes, transient ischaemic attacks (TIA) or thromboembolic complications in patients with HALT. In the series reported by Makkar et al, there was no difference in the incidence of stroke/TIA or thromboembolic complications in the PORTICO clinical trial; however, the presence of reduced leaflet motion was associated with a significant increase in the risk of TIA in the registries2. Nonetheless, as pointed out by the authors, the finding of increased risk of TIA associated with reduced leaflet motion is preliminary and inconclusive, being based on a small number of neurologic events observed in the study. Additional studies are needed to evaluate the impact of this finding on clinical outcomes.
Clinical implications
The fact that HALT/RELM is not observed in anticoagulated patients and resolves with initiation of anticoagulation suggests that this finding is related to THV thrombosis. In view of the available evidence, it may be reasonable to perform CT imaging in appropriate clinical situations (elevated aortic valve gradients, worsening heart failure, stroke/TIA, myocardial infarction or other clinical situations suggesting embolic phenomena) and anticoagulate patients with symptomatic THV thrombosis (HALT/RELM) for one to three months. The decision to continue anticoagulant therapy for longer than one to three months should be made on a case-by-case basis, after balancing the benefits of anticoagulation against the risk of bleeding.
On the other hand, the appropriate management of HALT/RELM in the presence of normal aortic valve gradients (subclinical THV thrombosis) is unclear. There are several clinically relevant issues that remain unresolved: (1) there is insufficient evidence to recommend routine CT screening for detection of subclinical THV thrombosis; (2) routine CT screening is expensive and carries the risk of overtreatment of artefact, radiation exposure and contrast-induced nephropathy; (3) the impact of subclinical THV thrombosis and its treatment on clinical outcomes is unknown; (4) the optimal timing of initiation, duration or type of anticoagulation therapy and the role of prophylactic anticoagulation for prevention of THV thrombosis are unclear; and (5) predictors of subclinical THV thrombosis (including the propensity of individual valve types) are unknown. Well-designed prospective studies evaluating the incidence, predictors and clinical impact of subclinical THV thrombosis are therefore required to address these important issues.
Although subclinical THV thrombosis is observed relatively frequently, routine anticoagulation of all patients with biological valves cannot be recommended at the present time; there are no conclusive data concerning clinical outcomes, and the risk of bleeding in a predominantly elderly population with multiple comorbidities could be high. Despite the lack of randomised clinical trials and the findings of a few smaller series reporting no benefit with routine anticoagulation after bioprosthetic SAVR11,12, the two largest series (Danish registry of 4,075 patients and Society of Thoracic Surgeons Adult Cardiac Surgery Database of 25,656 patients) reported a reduction in the incidence of death and embolic events with routine anticoagulation13,14. In a series of 1,521 patients undergoing TAVR, no anticoagulation at discharge post TAVR was associated with haemodynamic degeneration (defined as a 10 mmHg rise in aortic valve gradients) of the THV on follow-up echocardiography15. In a series of 397 consecutive explanted bioprosthetic valves, bioprosthetic valve thrombosis was noted in 46 (10%) cases and predicted by a subtherapeutic international normalised ratio (INR)16. The ACC/AHA and ESC guidelines provide a Class IIb recommendation for DAPT but do not recommend routine anticoagulation after TAVR. Prospective studies evaluating the relevance of early anticoagulation after TAVR are needed, especially since the indications for TAVR are expanding into lower-risk populations who may be more safely treated with anticoagulants.
The impact of this finding on long-term valve durability is unknown and may only be evident when this technology is used in younger patients with longer life expectancy. Until we have concrete evidence that this imaging finding is truly relevant, the management of patients with TAVR should be guided by randomised trials and registries focused on clinical outcomes. While it is important to study this finding further, it is also prudent to avoid overreaction, as stated in the recently published FDA perspective: “Whether reduced leaflet motion is clinically meaningful or represents a subclinical advanced-imaging phenomenon, the loss of leaflet mobility renders the valve dysfunctional and demands additional investigation. We at the FDA believe that the available clinical evidence supports the conclusion that these valves remain safe and effective and that findings to date concerning reduced leaflet motion have not changed the overall favorable benefit–risk balance for these valves when they are used for their approved indications”17.
Conflict of interest statement
R. Makkar has received grant support from Edwards Lifesciences, Medtronic and St. Jude Medical, and holds equity in Entourage Medical. H. Jilaihawi has received a consulting fee from Edwards Lifesciences, St. Jude Medical and Venus MedTech. T. Chakravarty and Y. Abramowitz have no conflicts of interest to declare.

