
Abstract
Aims: The aim of this study was to report the predictors and clinical impact of transcatheter heart valve (THV) thrombosis in patients undergoing transcatheter mitral valve implantation (TMVI).
Methods and results: We included 130 patients who consecutively underwent TMVI. Transoesophageal echocardiography (TOE) and/or computed tomography (CT) were performed in 91.7% of patients at discharge, in 73.3% at three months and in 72% beyond three months. THV thrombosis was defined as the presence of at least one thickened leaflet with restricted motion confirmed by TOE or contrast CT and classified as immediate, early, or late according to the timing of diagnosis. THV thrombosis was observed in 16 (12.3%) patients: immediate in 43.7%, early in 37.5% and late in 18.8%. Most of these thromboses were subclinical (93.7%) and non-obstructive (87.5%). No thromboembolic event occurred. After optimisation of antithrombotic treatment, THV thromboses resolved in all but one patient. Predictors were shock for immediate (p<0.001), male sex for early (p=0.045) and absence of anticoagulation for both early (p=0.018) and late (p=0.023) THV thromboses.
Conclusions: THV thrombosis is frequent after TMVI, occurs mainly within the first three months, is mostly subclinical and resolves after optimisation of antithrombotic treatment. An anticoagulation therapy for at least three months after the procedure is mandatory.
Introduction
Transcatheter mitral valve implantation (TMVI) using balloon-expandable aortic valves has been proposed as an alternative to surgery in high-risk patients with degenerated bioprostheses (valve-in-valve [ViV]), failed annuloplasty rings (valve-in-ring [ViR]) and severe mitral annular calcification (valve-in-mitral annulus calcification [ViMAC]). Despite limited evidence, preliminary results suggest that TMVI is feasible and safe when performed by experienced operators, with acceptable long-term clinical and haemodynamic outcomes1,2. However, TMVI is associated with several complications. While the risks of left ventricular outflow tract obstruction, valve migration or embolisation and paravalvular mitral regurgitation have been evaluated previously, uncertainty exists about the risk of transcatheter heart valve (THV) thrombosis3,4,5,6,7. Its incidence and clinical impact remain unclear and there is a lack of data about the management of this complication8.
We aimed to report the predictors and clinical impact of THV thrombosis after TMVI using balloon-expandable THVs.
Methods
DATA SOURCE AND POPULATION
By way of a prospective observational study, we evaluated all the patients consecutively treated with TMVI between July 2010 and September 2019 at our centre. We excluded two patients in whom the THV was not implanted. Indications for TMVI were severe symptomatic mitral valve disease due to bioprosthesis or ring failure or severe MAC in high-risk or inoperable patients in whom the Heart Team favoured percutaneous intervention over surgery. A small subset of the population consisted of young women with a desire for pregnancy in whom TMVI was considered to avoid or delay a surgical reintervention (which should have been bioprosthesis implantation in these specific cases).
Baseline characteristics, procedural data, echocardiographic/computed tomography (CT) findings and clinical outcomes including follow-up data for all patients were prospectively collected in a local database using the REDCap platform. Clinical outcomes were defined according to the Mitral Valve Academic Research Consortium (MVARC) criteria9. All patients signed consent forms stating their agreement to have their medical data recorded in a computer database for research purposes. This study was conducted in accordance with the principles of the Declaration of Helsinki and carried out in accordance with the local legislation.
TRANSCATHETER MITRAL VALVE IMPLANTATION
All patients treated with TMVI had systematic echocardiography (transthoracic [TTE] and transoesophageal [TOE]) and cardiac CT before the procedure to detect any contraindication to TMVI. Patients whose initial valve dysfunction was related to thrombosis were not referred to TMVI and treated with anticoagulation. The TMVI procedures have already been described2,10. Briefly, they were performed by a team of interventional cardiologists, under general anaesthesia, with TOE and fluoroscopy guidance and using the balloon-expandable SAPIEN XT until 2016 and then SAPIEN 3 THVs (both Edwards Lifesciences, Irvine, CA, USA). The default approach was transseptal. TOE was also mandatory to evaluate the immediate result and detect any complication. In case of paravalvular leak, the decision on post-dilation or implantation of a second THV relied on its mechanism (incomplete expansion or malposition) after careful analysis.
ANTITHROMBOTIC TREATMENT
Anticoagulation with intravenous heparin was administered at the beginning of the procedures (70 IU/kg for transseptal or transapical TMVI and 300 IU/kg for transatrial “hybrid” TMVI) and was restarted two hours after transseptal and six hours after transapical and transatrial procedures in the absence of complication with target anti-factor Xa activity of 0.4 to 0.7 IU/ml. In addition, antiplatelet therapy with aspirin 75 mg/day was initiated before the procedure. The combination of vitamin K antagonists (VKA) with a target international normalised ratio (INR) of 2-3 and aspirin 75 mg/day was indicated for the first three months in the absence of prohibitive bleeding risk. VKA were stopped thereafter if THV thrombosis was not diagnosed at the three-month visit and if patients had no other indication for long-term anticoagulation therapy. Aspirin was stopped after three months in patients requiring long-term anticoagulation for another indication. In patients with THV thrombosis observed after hospital discharge, antithrombotic therapy was reinforced and VKA were continued.
FOLLOW-UP
A clinical, echocardiographic and CT follow-up was planned at discharge, three months (median 72 [44-99] days), one year (median 417 [367-468] days) and yearly thereafter. All had a TTE at each visit, 115/130 (88.5%) had at least one TOE during the follow-up and 110/130 (84.6%) at least one cardiac CT. TOE and/or cardiac CT were performed in 91.7% of patients at discharge, in 73.3% at three months and in 72% beyond three months.
Echocardiography assessments were performed with a Philips iE33 until 2014 and with a Philips EPIQ 7 ultrasound machine thereafter (Philips Healthcare, Andover, MA, USA). The mean transmitral gradient calculated using the Bernoulli formula, and mitral valve area using the continuity equation were evaluated with TTE. Mitral regurgitation was graded as 1 (trace), 2 (mild), 3 (moderate), or 4 (severe) according to its severity. A complementary assessment by TOE and contrast CT was scheduled in the absence of contraindication.
Cardiac CT was retrospectively gated with data acquisition in all phases of the cardiac cycle and contrast enhancement, as previously recommended10.
DEFINITION OF THV THROMBOSIS
THV thrombosis was defined as the presence of at least one thickened leaflet with restricted motion or a mobile mass confirmed by TOE or cardiac CT, which also showed typical images of hypoattenuated leaflet thickening (HALT). It was classified according to the timing of diagnosis as immediate (until discharge), early (after discharge, until three months), or late (beyond three months). Clinical and haemodynamic parameters were not necessary to withhold the diagnosis, in accordance with the MVARC definitions9.
STATISTICAL ANALYSIS
Categorical variables are presented as frequencies and continuous variables as mean (standard deviation) or median (interquartile range) according to distribution. Comparisons between qualitative variables were made using the chi-square or Fisher’s exact test, as appropriate. Continuous variables were compared using a two-sample t-test or Wilcoxon rank-sum test, as appropriate. Logistic regression models were constructed to determine predictors of THV thrombosis at each time - immediate, early and late. Variables with a p-value <0.1 in the univariate analysis were included in the multivariate analysis. When analysing the predictors of immediate and late thrombosis, only one variable had a p-value <0.1 in the univariate analysis. Therefore, multivariate analyses were not performed for immediate and late thrombosis. Results are presented as odds ratios (OR) with their confidence interval (CI). All tests were two-sided and conducted at a 0.05 level of significance. Statistical analyses were performed using JMP software, version 15.0 (SAS Institute Inc., Cary, NC, USA).
Results
A total of 130 patients were included (Figure 1). At a median follow-up of 1.8 (0.9-3.5) years, 16 (12.3%) patients had a THV thrombosis. The one-year cumulative rate of THV thrombosis was 11.1% (95% CI: 6.5% to 18.2%). Immediate THV thrombosis was observed in 7 (43.7%) patients, early in 6 (37.5%) and late in 3 (18.8%). The median time from index procedure to diagnosis of thrombosis was 78 days (IQR 12-109).
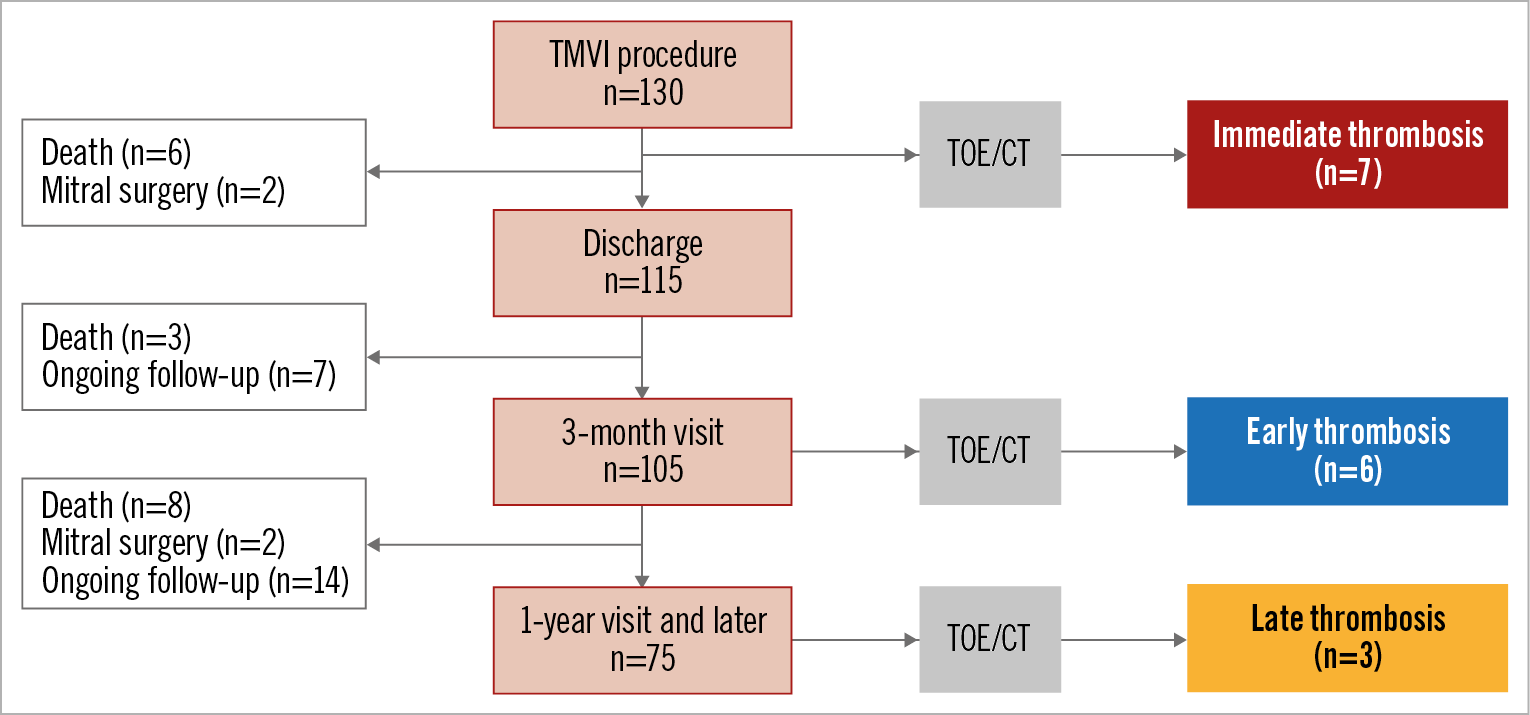
Figure 1. Study flow chart. CT: computed tomography; TMVI: transcatheter mitral valve implantation; TOE: transoesophageal echocardiography
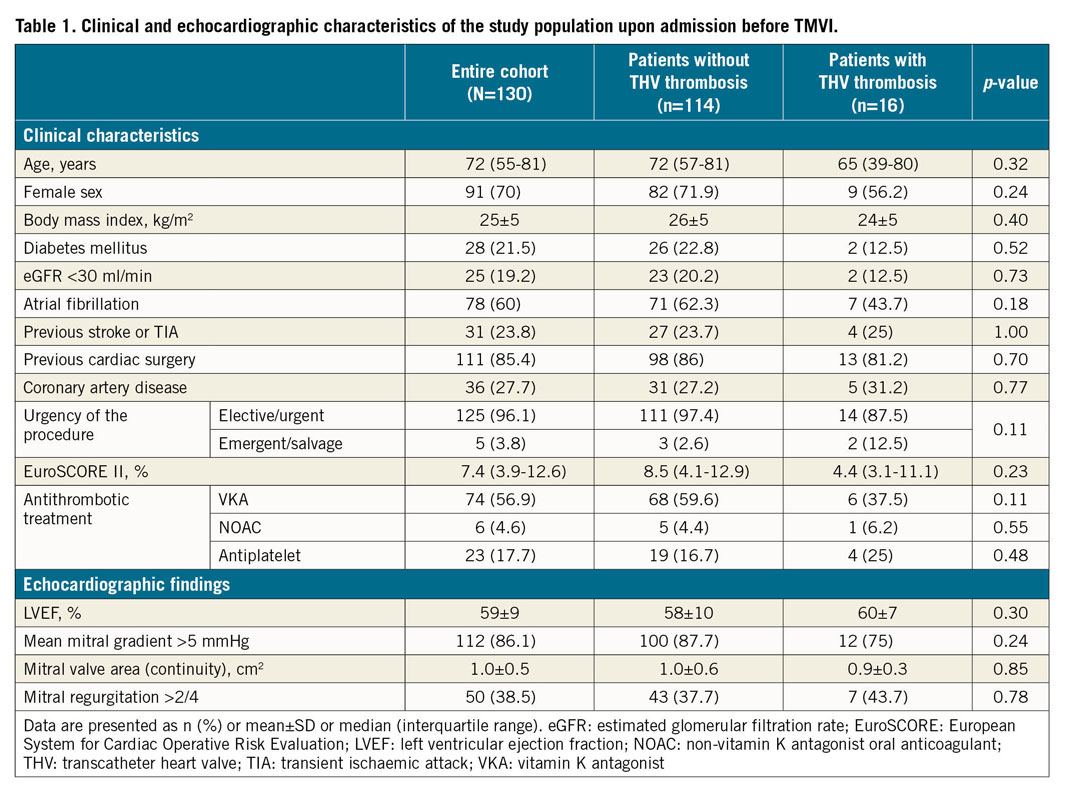
Clinical and echocardiographic characteristics of the overall population at baseline (upon admission before TMVI) are shown in Table 1. The median age of patients was 72 (55-81) years, they were more frequently women (70%) and were deemed at high surgical risk with a median EuroSCORE II of 7.4% (3.9-12.6%). There was no significant difference between patients with THV thrombosis and patients without. Patients with a THV thrombosis tended to be more frequently men (43.8% vs 28.1%, p=0.24), at lower surgical risk as reflected by the EuroSCORE II (4.4% [3.1-11.1%] vs 8.5% [4.1-12.9%], p=0.23), and less frequently to have atrial fibrillation (43.7% vs 62.3%, p=0.18) and thus less anticoagulation therapy before procedures (43.7% vs 64%, p=0.17). A salvage TMVI was performed in 2 (12.5%) patients with a THV thrombosis compared with 3 (2.6%) in patients without (p=0.11).
Procedural findings and 30-day outcomes in the overall population and according to the occurrence of THV thrombosis are listed in Table 2. There was no significant difference between groups. The procedure was a ViV TMVI in 47.6% of patients, ViR in 26.2% and ViMAC in 26.2%. The approach was transseptal in 90.8% of the patients and the most frequently implanted THV in both groups was the SAPIEN 3 (71.5%). Twenty-five percent (25%) of the patients with and 9.6% without THV thrombosis needed a second valve implantation during the index procedure (p=0.09).
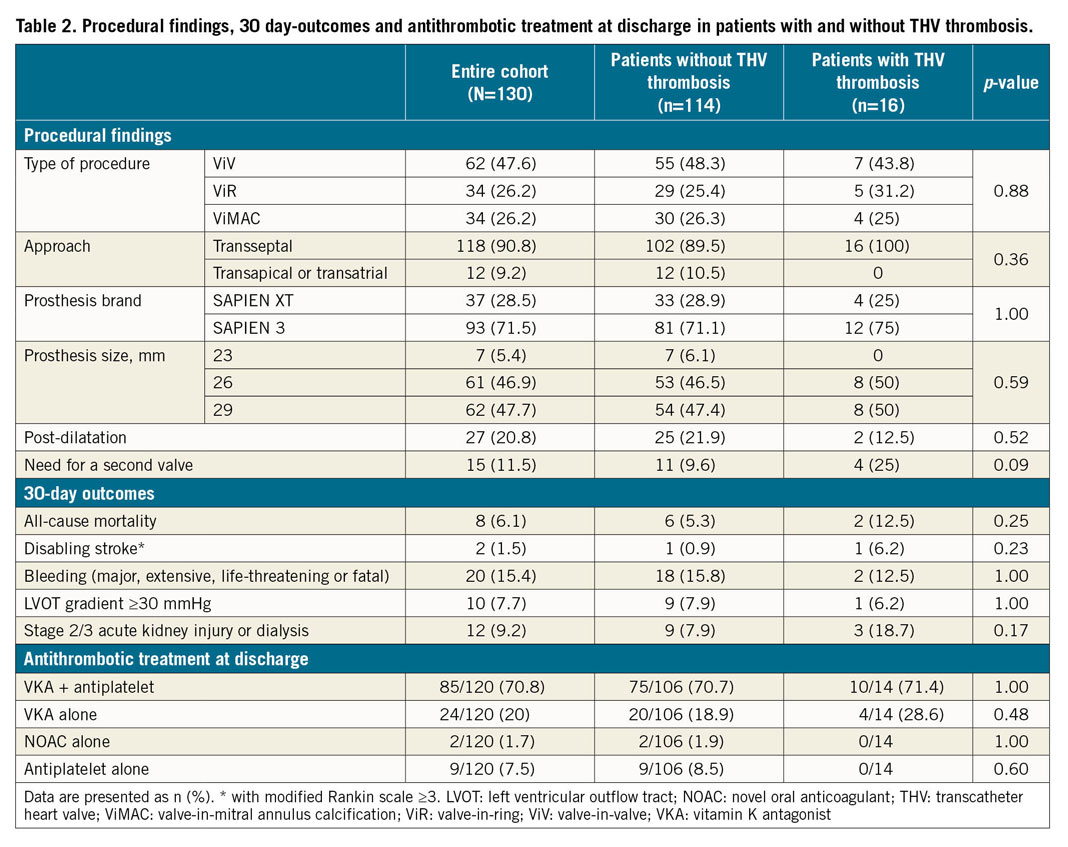
Regarding 30-day outcomes, 8 (6.1%) patients died: 2 (12.5%) in the group of patients with THV thrombosis and 6 (5.3%) in those without (p=0.25). Both patients with THV thrombosis died from non-related causes: one death was due to a septic shock and the other to a refractory multiorgan failure despite successful salvage TMVI in the context of cardiogenic shock. There was neither stroke nor any other thromboembolic event related to THV thrombosis. Data regarding antithrombotic treatment prescribed at discharge are listed in Table 2. A total of 111/120 patients (92.5%) were treated with anticoagulation treatment at discharge and 9/120 (7.5%) with antiplatelet alone, without difference between the groups. Among patients treated with anticoagulation at discharge and with a three-month follow-up visit, 8/103 (7.8%) discontinued their anticoagulation treatment (5 patients for unknown reasons and 3 due to bleeding concerns).
In patients with THV thrombosis, the mean transprosthetic mitral gradient was 7±2 mmHg. The mean delta transprosthetic gradient was 2.1±1.8 mmHg. A transprosthetic gradient >10 mmHg was observed in two patients and one of them reported worsening dyspnoea. The functional status of all the others remained unchanged. TOE was carried out in all. Cardiac CT examination was performed in 13/16 patients (three had severe kidney disease contraindicating CT). TOE missed 2/16 THV thrombosis (12.5%) while CT showed HALT associated with a restriction of cusp mobility in all of them. No case of thickened leaflet without restriction of mobility occurred. Thus, 11/16 (68.8%) thromboses were diagnosed by both TOE and cardiac CT, 3/16 (18.8%) by TOE alone and 2/16 (12.5%) by CT alone. Recurrence of THV thrombosis was observed in only one patient who had immediate THV thrombosis. It occurred six months after the first one in a pregnancy context. Individual characteristics of patients with THV thrombosis are shown in Supplementary Table 1.
Upon diagnosis of THV thrombosis, patients were treated with VKA in combination with antiplatelet therapy except for two patients who received VKA alone because of a prohibitive bleeding risk. At a median follow-up of 99 days after the diagnosis, thrombosis resolved in all but one patient who remained asymptomatic despite the persistence of a non-obstructive thrombosis confirmed by TOE and cardiac CT.
Predictive factors for THV thrombosis are listed in Table 3. Periprocedural shock was the only predictive factor of immediate THV thrombosis in univariate analysis (OR 41.4, 95% CI: 6.79-252.78, p<0.001). In univariate analysis, early THV thrombosis was associated with male sex, need for a second prosthesis during the index procedure and absence of anticoagulation treatment at the three-month visit. After adjustment, male sex (OR 10.41, 95% CI: 1.05-103.08, p=0.045) and the absence of anticoagulation (OR 12.37, 95% CI: 1.53-98.45, p=0.018) were independently associated with early THV thrombosis, while the need for a second prosthesis was no longer significant (OR 7.67, 95% CI: 0.72-81.22, p=0.09). Regarding late thrombosis, no patient with thrombosis was previously treated with anticoagulation, whereas 73.6% of patients without thrombosis were (Figure 2). Timing to THV thrombosis according to the procedure type is summarised in Figure 3.
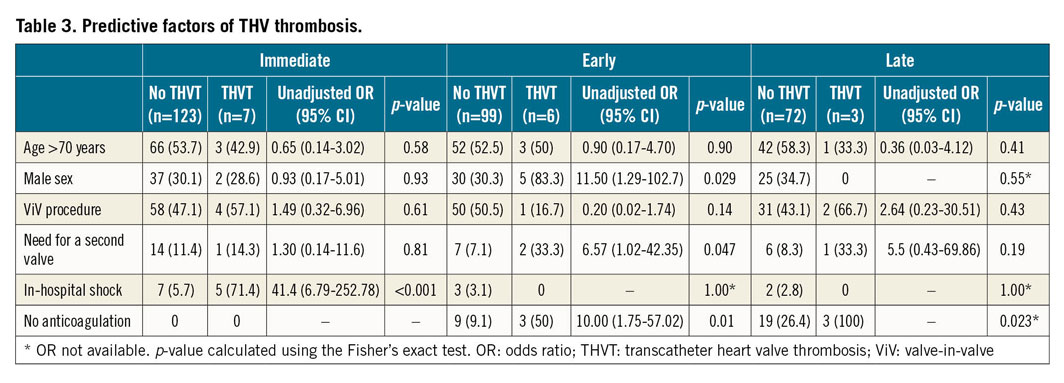
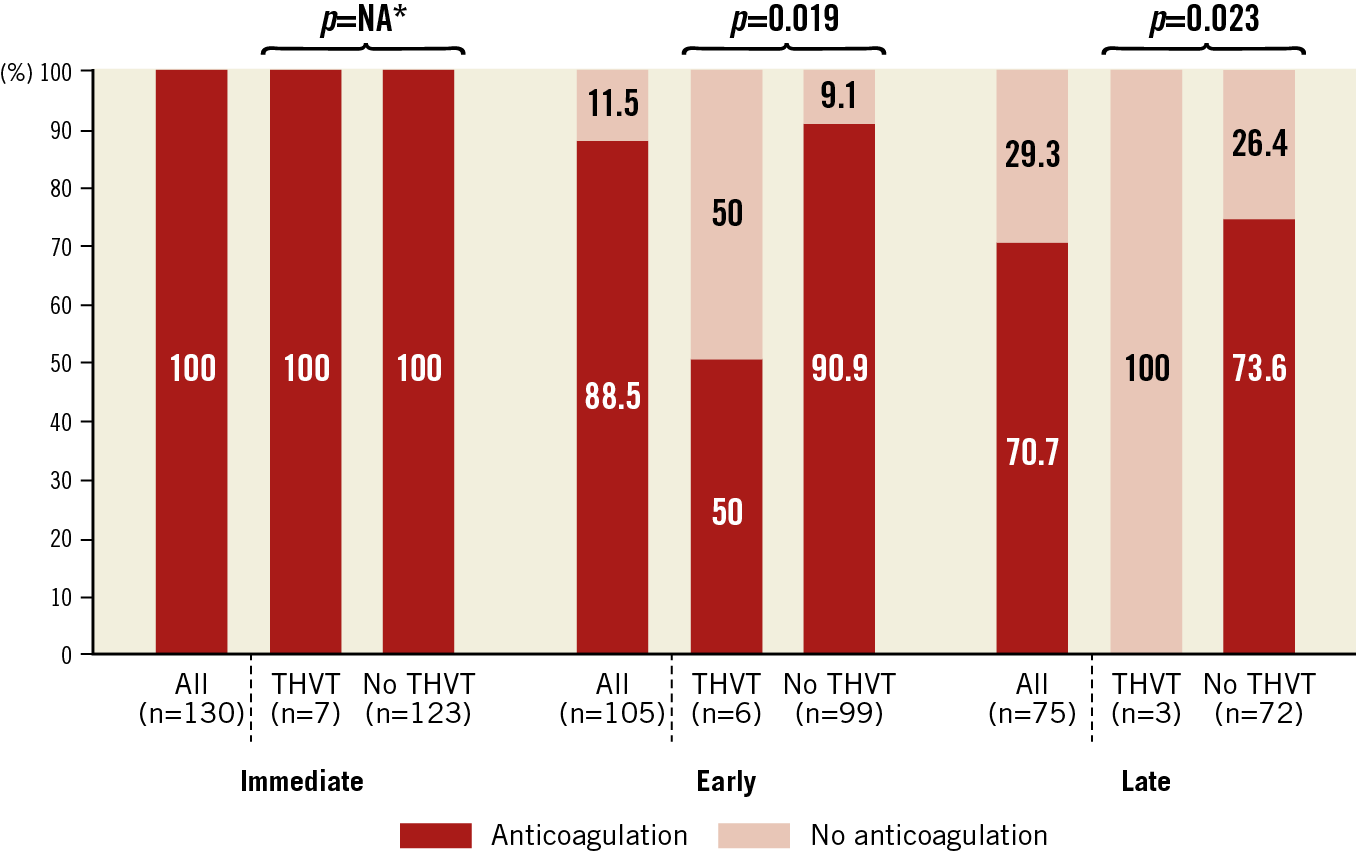
Figure 2. Rates of patients with anticoagulation treatment according to study groups at different time points. Comparisons between groups were made using the Fisher’s exact test. * non available: all patients received at least intravenous heparin after the procedure as described in the Methods section. Anticoagulation management was left to the discretion of each physician. THVT: transcatheter heart valve thrombosis
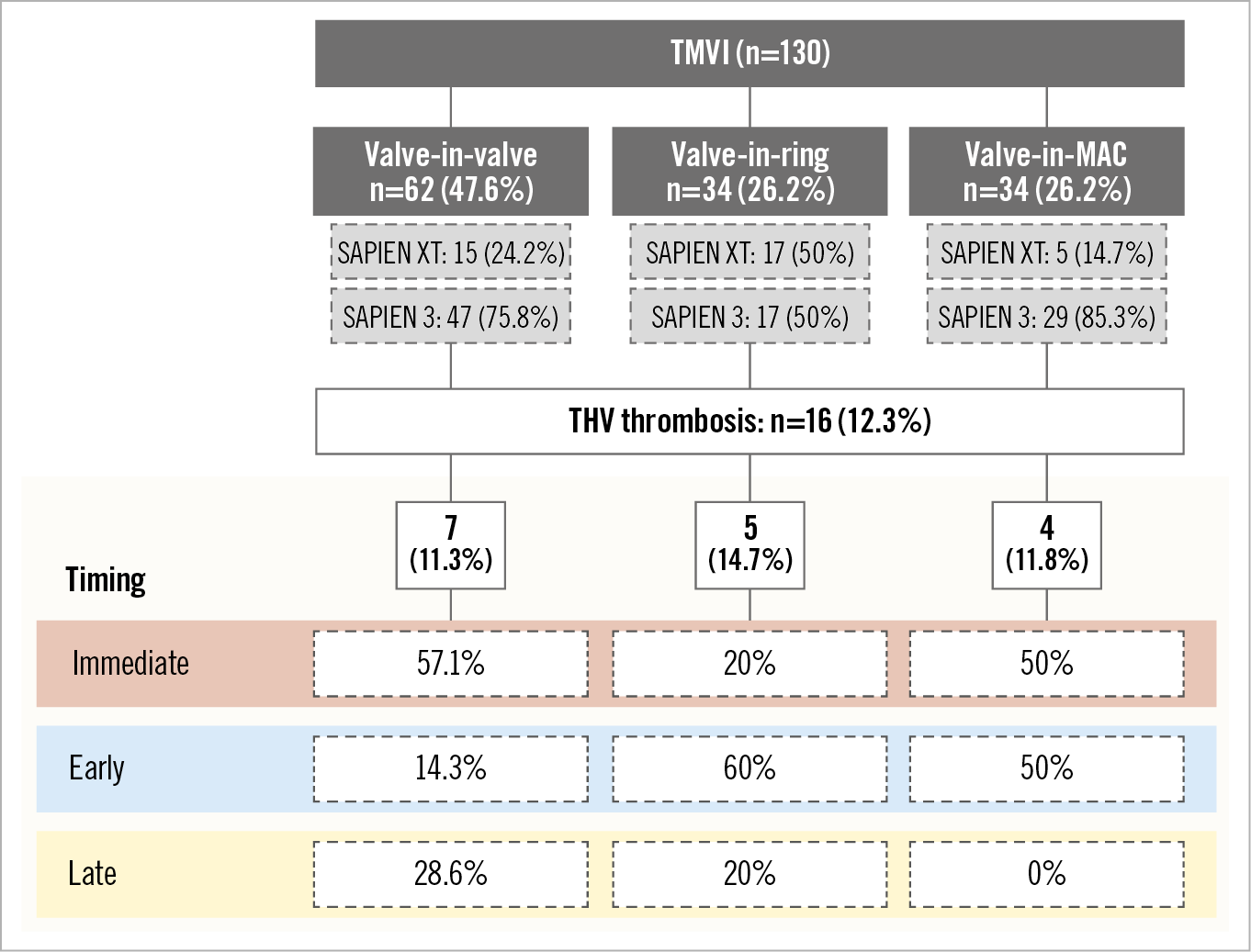
Figure 3. Timing to THV thrombosis according to the procedure type. MAC: mitral annulus calcification; THV: transcatheter heart valve; TMVI: transcatheter mitral valve implantation
Discussion
The main results of this study are: 1) THV thrombosis is frequent after TMVI and occurs mainly within the first three months after the procedure in patients not receiving therapeutic anticoagulation, 2) it is mostly subclinical, and 3) it resolves under optimal antithrombotic treatment.
This is the first study reporting the predictors and clinical impact of THV thrombosis in a large monocentric cohort of patients treated with TMVI undergoing TOE and cardiac CT. Yoon et al1 recently published data from a large international multicentre TMVI registry. Information on THV thrombosis was available in 411/521 (78.9%) patients. It was observed in 11 (2.7%) patients. As in the present study, patients without anticoagulation had a higher rate of thrombosis at one year than those with anticoagulation (6.6% vs 1.6%, p=0.019), supporting the preventive effect of anticoagulation. However, the one-year cumulative rate of THV thrombosis was far higher in our study. This may be due to two main reasons: 1) we used the MVARC definitions, contrary to Yoon et al who attributed “a posteriori” valve dysfunction to THV thrombosis on the basis of the response to anticoagulation or surgical findings; 2) we used cardiac CT and echocardiography to increase the sensitivity of the diagnosis of subclinical thromboses.
Importantly, most patients were asymptomatic at the time of diagnosis, highlighting the relevance of systematic follow-up with TTE. The high rate of subclinical thromboses reported in our study is explained mainly by their detection using multimodality imaging (echo, CT). Patients with THV thrombosis have an excellent prognosis when diagnosed at an early stage and treated with optimal antithrombotic treatment. Nevertheless, questions about the immediate and long-term clinical consequences of untreated subclinical THV thrombosis remain open11,12,13. Whether routine screening with TOE and CT would be justified in clinical practice remains unknown. It has been suggested in transcatheter aortic valve replacement that THV thrombosis might be an early phase of valve degeneration14. Whether this is true for TMVI is unknown. We did not observe any prosthesis degeneration in our cohort of TMVI patients in this study but the duration of follow-up was relatively short. Despite the absence of major clinical consequences, a closer clinical and TTE follow-up in patients with THV thrombosis seems mandatory.
The present results show that THV thrombosis occurs mainly within the first three months after the procedure in patients not receiving anticoagulation. During the endothelialisation period, biological processes such as formation of a platelet–fibrin network on the prosthetic surface predispose to thrombosis15,16. Thus, anticoagulation is essential during this period. Whether it should be continued beyond three months remains an open question, although it surely depends on the bleeding risk of each patient. Moreover, its routine combination with antiplatelet therapy is questionable. Despite the limited data about bleedings in patients treated with TMVI1,2, the risk is probably high due to the overall severity of the patients’ profiles. The assessment of each patient’s risk-benefit ratio should be repeated at each follow-up visit to adapt the nature and degree of antithrombotic therapy. In our study, 7.8% of patients treated with VKA at discharge had discontinued their treatment before the three-month follow-up visit. Although this rate is comparable to previously reported data17, strategies should be implemented to improve the adherence to anticoagulation therapy. In addition to therapeutic education, healthcare facilities dedicated to monitoring of anticoagulation therapy might be helpful.
Periprocedural shock may be considered a risk factor for immediate THV thrombosis. The low cardiac output in cardiogenic shock favours hypercoagulability and thrombotic events by increasing blood stasis16. Also, we observed a higher incidence of early THV thrombosis in men than in women. Although other studies have reported the same finding in patients with thrombosis after transcatheter aortic valve replacement11,18, the reason is unclear. It may simply be related to the use of multiple statistical comparisons on a small study sample.
Despite the absence of definitive statistical evidence, the need for a second prosthesis during the index procedure could be a factor promoting thrombosis (p=0.09 in multivariate analysis). It is likely that the implantation of a second prosthesis increases the local metallic burden and delays the endothelialisation process, resulting in an increased risk of thrombosis.
Limitations
Although the present analysis involves a large cohort of patients treated with TMVI, it is a single-centre observational study without a central adjudication committee. The rate of THV thrombosis may have been underestimated by the competing risk of death (patients who died suddenly or from uncertain causes may have had undiagnosed thrombosis) and by the absence of TOE or CT data in some patients. Indeed, some patients did not undergo these exams mainly because of contraindication (renal failure, highly frail patient, history of thoracic radiation), and to a lesser extent for logistical reasons, since 11.5% of patients were followed after discharge in other institutions which did not routinely perform TOE or cardiac CT at each visit. Since this is an observational study, the presence of potential confounding factors cannot be ruled out. In addition, periods of subtherapeutic INR may have occurred in patients treated with VKA without THV thrombosis. From a statistical standpoint, the low number of events resulted in a lack of power. The results of the multivariate analysis should be interpreted with caution.
Conclusions
THV thrombosis is frequent after TMVI. It occurs mainly within the first three months after the procedure, is mostly subclinical and resolves after optimisation of antithrombotic treatment. Anticoagulation seems mandatory immediately after TMVI and might be necessary beyond three months although its duration depends on the bleeding risk of each patient. The results of this hypothesis-generating study need to be supported by further larger studies.
|
Impact on daily practice THV thrombosis is frequent after TMVI. Prophylactic anticoagulation with VKA should be considered in all patients after the procedure. A treatment duration of at least three months is necessary after assessment of the individual risk-benefit ratio. |
Conflict of interest statement
A. Vahanian has received consultancy fees from CardioValve. B. Iung has received consultancy fees from Edwards Lifesciences and travel fees from Boehringer Ingelheim. D. Himbert has received proctoring fees from Edwards Lifesciences and Abbott Vascular. E. Brochet has received proctoring fees from Abbott Vascular. G. Ducrocq has received consultancy fees from Amgen, AstraZeneca, Bayer, BMS, Janssen, Sanofi, and Terumo and proctoring fees from Boston Scientific, and Novo Nordisk. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

