- annulus
- aortic regurgitation
- aortic stenosis
- CT
- transcatheter aortic valve implantation
Abstract
Aims: Transoesophageal echocardiography (TEE) is considered the gold standard method for annulus measurement in transcatheter aortic valve implantation (TAVI). However, computed tomography (CT) has potential advantages compared to TEE. We sought to assess the impact of CT-guided valve sizing on post-procedural aortic regurgitation (AR).
Methods and results: We compared procedural characteristics and clinical outcomes in patients undergoing either TEE-guided or CT-guided TAVI. Among 350 consecutive TAVI recipients, the mean age was 83.2±6.4 years and the logistic EuroSCORE was 22.4±11.2%. The mean Diam-TEE was similar in both groups (22.3±1.9 mm vs. .0±1.8 mm, p=0.092). The mean annulus diameter by CT (mDiam-CT) was larger than mean Diam-TEE (23.6±2.0 mm vs. 22.3±1.9 mm, p<0.001), and resulted in larger valve implant sizes compared to the TEE-guided group (25.8±2.1 mm vs. 25.0±1.9 mm, p<0.001). The incidence of post-procedural AR ≥grade 2 was significantly reduced in the CT-guided group (15.4% vs. 24.0%, p=0.044), with a similar risk of annulus rupture (0.6% vs. 1.7%, p=0.31). The only predictor of post-procedural AR ≥2 was the “valve/mDiam-CT ratio” (HR 0.36 by increase of 0.1, 95% CI: 0.17-0.77, p=0.008) by multivariate analysis.
Conclusion: CT-guided valve sizing in TAVI significantly reduces the incidence of post-procedural AR compared to TEE sizing. This strategy may have the potential to improve clinical outcomes.
Abbreviations
AS: aortic stenosis
BMI: body mass index
CABG: coronary artery bypass graft
CI: confidence interval
COPD: chronic obstructive pulmonary disease
Diam-CT: annulus diameter measured by CT
Diam-TEE: annulus diameter measured by transoesophageal echocardiography
eGFR: estimated glomerular filtration rate
HU: Hounsfield units
LVEF: left ventricular ejection fraction
lDiam-CT: long-axis CT-measured annulus diameter
mDiam-CT: CT-measured geometric mean annulus diameter
MI: myocardial infarction
MDCT: multidetector computed tomography
MIP: maximal intensity projection
NYHA: New York Heart Association
PCI: percutaneous coronary intervention
ROC: receiver operating curve
ROI: region of interest
sDiam-CT: short-axis CT-measured annulus diameter
TAVI: transcatheter aortic valve implantation
TEE: transoesophageal echocardiography
VARC: Valve Academic Research Consortium
Valve/Diam-
TEE ratio ratio between the valve size and Diam-TEE
Introduction
Transcatheter aortic valve implantation (TAVI) is a viable therapeutic option for patients with severe symptomatic aortic stenosis (AS) who are ineligible or high-risk for conventional surgical aortic valve replacement1-3. Although this technique has reached relative maturity, further optimisation of patient selection and device implantation is necessary. Accurate measurement of the aortic annulus is crucial for appropriate valve sizing in TAVI. Overestimation of the annulus size can cause catastrophic annulus rupture, while underestimation can result in valve migration or post-procedural paraprosthetic aortic regurgitation (AR), a new predictor of 30-day4 and long-term mortality in TAVI5.
The aortic valve is a complex three-dimensional structure with the valve annulus usually used to describe the anatomic ventriculo-aortic junction. However, the aortic annulus is not a distinct anatomic structure, and thus the virtual ring, which is formed by the junction of the nadirs of all aortic valve leaflets at the distal part of the left ventricular outflow tract, is used to describe the ventriculo-aortic junction during TAVI6,7.
Measurement of the diameter of aortic annulus for valve prosthesis sizing for TAVI is historically and most commonly performed using transoesophageal echocardiography (TEE)7-10. However, there remains considerable regional variation in the use of TEE for valve sizing, and the accuracy of this technique may be limited by the one-dimensional nature of sagittal measurements in an oval, three-dimensional structure with variable orientation6,7,11-15. Although three-dimensional (3-D) TEE circumvents many of the limitations of conventional TEE, it is currently not recommended for annulus measurement in TAVI10,15.
Multidetector computed tomography (MDCT) is an emerging non-invasive strategy for valve sizing in TAVI. Superior spatial resolution and 3-D measurements of the aortic annulus may improve valve sizing and could potentially improve patient outcomes6,7,11. Recently, two studies have demonstrated the superiority of CT-guided annulus sizing compared to TEE guidance, in regard to reducing paravalvular AR in TAVI using the Edwards SAPIEN balloon-expandable valve16,17.
The objective of this study was to compare the incidence of post-procedural AR From TEE-guided and CT-guided valve sizing in a large cohort of TAVI recipients.
Methods
STUDY POPULATION AND DESIGN
From October 2006, consecutive high-risk patients with symptomatic severe AS treated with TAVI at our institution were prospectively included in our TAVI database. Patients with symptomatic severe AS (valve area ≤1.0 cm2) were considered candidates for TAVI if they had a logistic European System for Cardiac Operative Risk Evaluation score (EuroSCORE) >20%, or if surgery was deemed to be of excessive risk due to significant comorbidities, or if other risk factors not captured by these scoring systems (e.g., porcelain aorta) were present. The decision to proceed with TAVI was discussed by a dedicated heart team, which included: experienced clinical and interventional cardiologists, cardiovascular surgeons and anaesthesiologists. All patients selected for TAVI underwent screening physical examination, transthoracic and transoesophageal echocardiography, baseline laboratory tests and coronary angiography. Assessment of the aortic annulus size was performed by TEE and/or MDCT.
Between October 2006 and October 2011, a total of 424 patients were included in our TAVI database. TEE-guided valve sizing was performed during our earlier experience and CT-guided sizing has been progressively introduced since 2009. In this analysis, six patients who did not receive a valve bioprosthesis in our early institutional experience (n=68, with 33 cases [48.5%] of post-procedural AR ≥2 and 1 case [1.5%] of annulus rupture) were excluded in order to minimise bias due to the learning curve. The remaining 350 cases are the subject of the current investigation. The patients in the CT-guided group (n=175) underwent aortic valve annulus assessment with both MSCT and TEE (Figure 1).
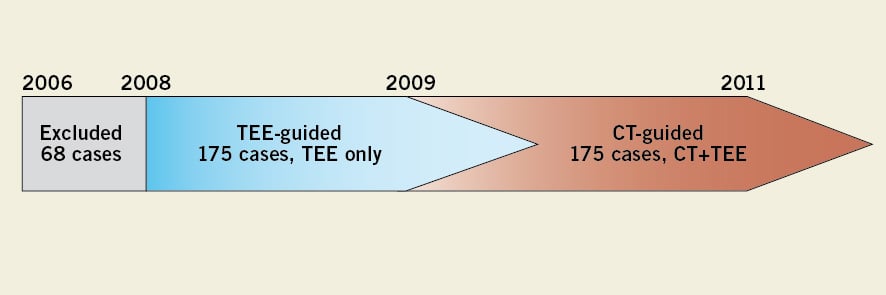
Figure 1. Study design. Our early institutional experience (n=68) was excluded from the current analysis, so as to minimise the effect of operator experience on outcomes. Initially, TEE was used for valve sizing, though CT-guided valve sizing was gradually introduced from 2009. Clinical outcomes were compared between the 175 cases that underwent TEE-guided valve sizing and the 175 cases that underwent CT-guided sizing. A further comparison of TEE and CT aortic annulus measurement was performed in the CT-guided group.
All patients agreed to participate in the study, and written informed consent was obtained in all cases.
VASCULAR ACCESS AND VALVE SELECTION
Patients were selected to undergo TAVI via the transfemoral approach or alternative approaches depending on the size, calcification and tortuosity of the ilio-femoral arterial access. The type of valve prosthesis was selected according to the diameter of the aortic annulus, which in our early experience was systematically measured using TEE (Diam-TEE), and more recently the mean annulus diameter has been calculated using MDCT (mDiam-CT). The Edwards valve (Edwards Lifesciences, Irvine, CA, USA) was used in patients with a diameter between 18 and 24.5 mm, and the CoreValve® (Medtronic, Inc., Minnneapolis, MN, USA) for annular diameters between 20 and 26.5 mm. For historical reasons (the Edwards valve was first introduced in 2006), the Edwards valve was used preferentially in patients with an annulus diameter between 20 and 24.5 mm, who were suitable for treatment with both valves. The CoreValve prosthesis was implanted in patients whose annulus size was >24.5 mm, or in patients with borderline ilio-femoral access precluding the use of 19, 22 or 24 Fr Edwards sheaths. The trans-subclavian or trans-aortic approach was used as an alternative in cases of unsuitable femoral arterial access in recipients of the CoreValve, and the transapical, trans-subclavian or trans-aortic route as the alternative to suboptimal femoral access with the Edwards valve. The same criteria for bioprosthesis sizing and selection were applied for both CT-guided and TEE-guided groups throughout the study period.
TRANSOESOPHAGEAL ECHOCARDIOGRAPHY
A detailed TEE study was performed in all cases during initial patient assessment by experienced echocardiographers using the Philips iE33 ultrasound system (Philips Medical, Amsterdam, The Netherlands) and a dedicated Philips TEE probe. The aortic annulus diameter was defined as the distance (mm) between the hinge points of the aortic valve leaflets, and was measured in the long-axis view of the aortic valve at end-systole, according to published recommendations10,18. In all cases, the diameter was measured three times and the mean value used for TEE valve sizing. The degree of preoperative and postoperative AR was measured using Doppler echocardiography18 and was defined according to the Valve Academic Research Consortium criteria19: 0) none, 1) trivial, 2) mild, 3) moderate, and 4) severe for quantitative analysis. Evaluation was performed by two experienced echocardiographers, who were blinded to the procedural information.
MULTIDETECTOR COMPUTED TOMOGRAPHY
All examinations were performed using a Philips Brilliance 64-slice MDCT scanner (Philips Medical). Standard technical parameters were used: gantry rotation time 330 ms, axial coverage 40 mm (64×0.625 mm), 120 kV tube voltage, 850-900 mAs intensity without modulation, temporal resolution 165 ms. Retrospective ECG gating was performed. Contrast enhancement was achieved with 50-80 ml of Iomeprol 400 mg/ml (Iomeron®). To achieve optimal synchronisation, a bolus tracking method was used in the descending aorta. Additional beta-blockade was not administered in any case, due to potential haemodynamic instability in severe AS. Only one case was unable to be examined by CT-scan due to high heart rate. The thickness of reconstructed images was 0.67 mm. All data were transferred to an offline post-processing dedicated workstation (EBW; Philips Medical).
The mid-systolic phase of the cardiac cycle was selected (20% or 30% of R-R interval) and measurement of the aortic annulus diameter was performed in the oblique plane that includes the nadirs of all three aortic cusps, and is perpendicular to the aortic root axis as previously described6,7. In this plane, the virtual annulus ring appears oval in shape, allowing two orthogonal diameters (long-axis and short-axis) to be measured (Figure 2A).
The annulus surface area was then manually traced with a caliper (Figure 2B) and the CT-measured geometric mean annulus diameter was derived as: mDiam-CT=2√ (annulus surface area/π). This value represents the average of all annulus diameters, according to a previously described method20. All measurements were performed in triplicate, and the mean value used for valve sizing.
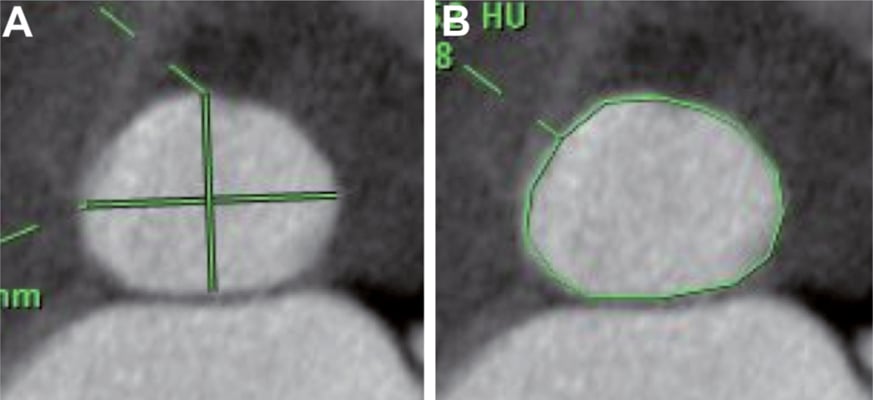
Figure 2. Measurement of an aortic annulus on MDCT. A) The surface area of the aortic annulus was measured as 493 mm2. Mean Diam-CT was calculated as 25.4 mm based on the formula: mean Diam-CT=2√(annulus surface/π). B) The short-axis and long-axis diameters were measured as 22.5 mm and 28.3 mm, respectively.
The degree of calcification of the aortic valve was evaluated in mid-diastole using a simple linear regression method between calcification and blood densities. A slice was positioned on the aortic valve, slice thickness increased to >20 mm, and maximal intensity projection (MIP) applied along the aortic root axis. A circular region of interest (ROI) including the whole valve and extending to the three aortic valve commissures allowed measure of mean density of the valve (D1) in Hounsfield units (HU); two other ROI of 100 mm2 positioned 10 mm below and 10 mm above the aortic valve were averaged to estimate the density of blood + contrast media at the level of aortic valve (D2). Then linear regression between D1, D2 and calcium density (1,000 HU) gave the degree of calcification of the aortic valve, expressed as a percentage of valve surface. This method was validated with a previously described semi-quantitative method for calcium quantification21 (r=0.85, p<0.001).
The calcification score of the virtual annulus ring was calculated as the sum of five local scores, each graded as 0 (no or mild calcification) or 1 (moderate or severe): 1) anterior leaflet of the mitral valve, 2) interatrial septum (right fibrous trigone), 3) membranous septum, 4) interventricular septum, 5) epicardial fat (left fibrous trigone).
PROCEDURES AND VALVE SIZING
Prior to TAVI, all patients were taking aspirin (160 mg) and clopidogrel (75 mg) daily, or were given a loading dose of clopidogrel (300-600 mg) before or immediately after the procedure. A bolus of intravenous heparin (70 IU/Kg) was administered at the start of each procedure in order to achieve an activated clotting time (ACT) of 250-300 seconds, and the ACT was measured every 30 minutes thereafter. The selected valve size for each patient was chosen based on the TEE results in the first 175 patients, and the MSCT in the second 175 patients. All procedures were performed by experienced interventional cardiologists according to our standard operating procedures, as previously described22.
POST-PROCEDURAL CARE
All patients were observed in the intensive care unit for at least 24 hours after Edwards valve implantation or 72 hours after CoreValve implantation (patients without previous pacemaker). Dual antiplatelet therapy was continued for six months and thereafter aspirin was continued indefinitely.
ENDPOINT DEFINITIONS
The primary endpoints of this study were related to complications of valve sizing, including post-procedural AR ≥2/4 and aortic annulus rupture. To evaluate the impact of the learning curve in the CT-guided group, the first 88 cases were defined as the “early experience group”. These patients were included in the multivariate analysis to identify the predictors of post-procedural aortic regurgitation ≥2 in the CT-guided group.
STATISTICAL ANALYSIS
Quantitative variables are expressed as mean ± standard deviation and qualitative variables as number and percentage. Comparison of quantitative variables was performed with an unpaired Student’s t-test or Wilcoxon rank sum test, depending on variable distribution. A paired t-test and Pearson correlations were used to compare the mDiam-CT and Diam-TEE in patients who underwent aortic annulus assessment with both modalities in the CT-guided group. The chi-square test or Fisher’s exact test was used to compare qualitative variables. Agreement and bias among modalities was assessed using Bland-Altman analysis. A stepwise logistic regression analysis, including all variables with p value ≤0.10 in the univariate analysis, was performed to determine the predictors of post-procedural aortic regurgitation ≥2/4 in the CT-guided group. Statistical significance was defined as p<0.05. The data were analysed with PASW statistics 17.0 (SPSS Inc., Chicago, IL, USA).
Results
PATIENT CHARACTERISTICS
Between October 2008 and October 2011, 350 patients received either the Edwards valve (n=297) or the CoreValve Revalving system (n=53) at our institution. The mean age of the entire population was 83.2±6.4 years (Table 1). Congestive heart failure class III/IV was prevalent in 84.9%, coronary artery disease in 58.6%, previous coronary artery bypass grafting (CABG) in 14.6%, and 66.3% of patients had significant renal dysfunction (eGFR <60 ml/min). The mean logistic EuroSCORE was 22.4±11.2%. The mean pressure gradient across the aortic valve was 47.6±16.5 mmHg, and the mean Diam-TEE was 22.1±1.9 mm.
In the CT-guided group, patients had a lower incidence of previous MI (7.4% vs. 14.9%, p=0.027) and had better renal function (eGFR <60 ml/min: 60.6% vs. 72.0%, p=0.024). The logistic EuroSCORE was lower in the CT-guided group compared to the TEE-guided group (20.1±10.4% vs. 24.4±11.5%, p=0.001). There was no significant difference between the groups in aortic valve area, gradient or baseline aortic regurgitation. Bicuspid valve was observed more frequently in the CT-guided group (8.6% vs. 0.6%, p<0.001).
AORTIC VALVE ASSESSMENT WITH MULTIDETECTOR CT AND TRANSOESOPHAGEAL ECHOCARDIOGRAPHY
Of the 175 patients who underwent both TEE and MDCT assessment of the aortic valve, 15 patients had an anatomically bicuspid valve (Table 2). Using MDCT, the short-axis diameter (sDiam-CT) was 21.8±2.0 mm, the long-axis diameter (lDiam-CT) was 26.4±2.6 mm, and the mean diameter (mDiam-CT) was 23.6±2.0 mm. The ratio between long-axis and short-axis diameters was 1.21±0.09. The mean aortic valve calcification degree was 28.8±11.7% and the mean aortic annulus calcification score was 0.71±0.77.
A comparison between CT and TEE measurements showed that the mDiam-CT was significantly larger than the Diam-TEE (23.6±2.0 mm vs. 22.3±1.9 mm, p<0.001).
The agreement between Diam-TEE and lDiam-CT (average bias 3.98 mm, 95% CI: –0.34 to 8.30 mm, Figure 3C) was worse than the agreement between Diam-TEE and mDiam-CT (average bias 1.25 mm, 95% CI: –1.93 to 4.43 mm, p<0.001, Figure 3A), or the sDiam-CT (average bias –0.5 mm, 95% CI: –3.56 to 2.56 mm, p<0.001, Figure 3B).
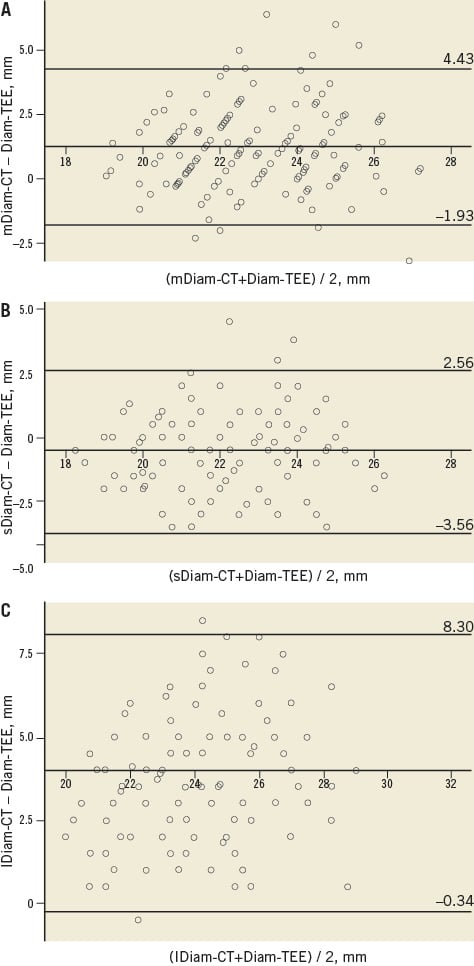
Figure 3. Bland-Altman analysis of Diam-TEE and Diam-CT. Bland-Altman plots demonstrating closer agreement and lower bias for Diam-TEE and mDiam-CT (A) and sDiam-CT (B) compared to lDiam-CT (C). The middle line represents the mean, the upper line +2 standard deviations (SDs) and the lower line -2 SDs. mDiam-CT: mean annulus diameter measured by CT; sDiam-CT: short-axis annulus diameter measured by CT; lDiam-TEE: long-axis annulus diameter measured by CT; Diam-TEE: annulus diameter measured by transoesophageal echocardiography
Based on the results of the MDCT assessment, the valve size selected for implantation was changed in 34 (19.4%) cases: valve upsizing in 31 patients (17.7%) and downsizing in three cases (1.7%).
PROCEDURAL CHARACTERISTICS
The Edwards valve was used in 84.9% of cases, the CoreValve in 15.1%, and the transfemoral approach was performed in 56.3% (Table 3). The most commonly used implant was the Edwards 26 mm valve (47.7%). The ratio of the valve size to the Diam-TEE (Valve/Diam-TEE ratio) was 1.15±0.09. Valve post-dilatation was performed in 10.9% of cases.
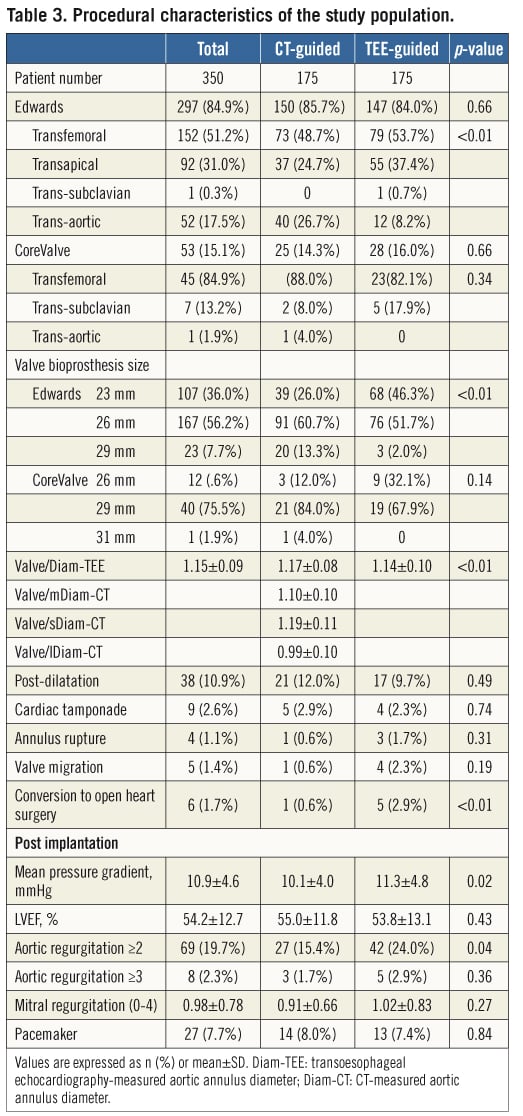
Serious complications were observed as follows: aortic annulus rupture in four patients (1.1%), valve migration in five (1.4%), and conversion to open aortic valve surgery in six cases (1.7%).
PROCEDURAL RESULTS
Post-procedural AR grade ≥2 was observed in 69 (19.7%) of patients, and occurred significantly less frequently in CT-guided patients compared to TEE-guided patients (15.4 vs. 24.0%, p=0.044). When the cohort of patients treated with the Edwards valve was considered in isolation, a similar trend towards a reduction in post-TAVI AR ≥2 with CT-guided sizing was observed (12.8% vs. 20.9%, p=0.059).
This reduction in AR ≥2 was mediated by larger valve sizes being implanted in CT-guided patients (25.8±2.1 mm vs. 25.0±1.9 mm, p=0.001). The valve/Diam-TEE ratio was also increased in the CT-guided group (1.17±0.08 mm vs. 1.14±0.10 mm, p=0.014) compared to the TEE-guided group. No difference in the incidence of annulus rupture was observed between the two strategies (0.6% vs. 1.7%, p=0.311). Following implantation, the mean aortic valve pressure gradient was 10.9±4.6 mmHg, and a lower aortic pressure gradient was also observed in CT-guided patients (10.1±4.0 mmHg vs. 11.3±4.8 mmHg, p=0.015).
PREDICTORS OF POST-PROCEDURAL AORTIC REGURGITATION
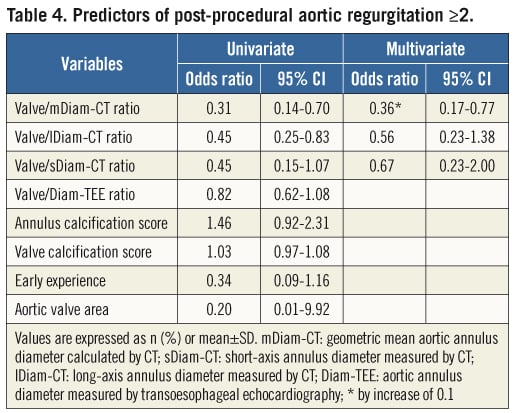
Predictors of post-procedural AR, including CT indices, were evaluated in the CT-guided group. Of note, the valve/Diam-TEE ratios, the annulus or valve calcification scores, early centre experience and aortic valve area were not associated with AR grade ≥2 by univariate analysis (Table 4). In contrast, the valve/mDiam-CT, valve/sDiam-CT and/or valve/lDiam-CT ratios were associated with post-implant AR by univariate analysis. Following adjustment for other variables, only the valve/mDiam-CT ratio was predictive of post-procedural AR (HR: 0.36 by increase of 0.1, 95% CI: 0.17-0.77).
The sensitivity-specificity curves identified a threshold of the valve/mDiam-CT ratio of 1.1, which best predicted post-procedural AR ≥2. With this cut point, the sensitivity, specificity, positive and negative predictive values were 58.1%, 63.2%, 25.4% and 87.5%, respectively. This valve/mDiam-CT ratio threshold predicted a higher incidence of post-procedural AR (25.4% vs. 12.5%, p=0.029).
Discussion
This study demonstrates that compared to traditional TEE valve sizing in TAVI, CT-guided sizing, using the mDiam-CT, results in larger valve size selection, a lower incidence of post-procedural AR ≥2, and a reduction in the mean aortic pressure gradient after valve implantation. Furthermore, in this large cohort of TAVI recipients, we describe the valve/mDiam-CT ratio as a novel independent predictor of post-procedural AR ≥2.
POTENTIAL ADVANTAGES OF CT-GUIDED AORTIC ANNULUS ASSESSMENT
Appropriate aortic valve sizing and precise implantation are of critical importance in TAVI. Determination of the appropriate valve size for implantation is based on the accurate assessment of the aortic annulus size; however, identification and measurement of this structure is problematical. It is recommended that valve sizing be performed by measuring the diameter of the virtual ring, the point of separation between the left ventricular outflow tract and the basal nadirs of the aortic valve leaflets6,7. Accurate sizing of the oval-shaped annulus should be performed in the transverse plane and perpendicular to the aortic root axis6,7,11.
To date, assessment of the aortic annulus diameter and subsequent valve sizing has been performed using two-dimensional (2-D) TEE in many centres7-10. However, given the oval shape and variable orientation, 2-D TEE may underestimate the true size of the aortic annulus6,7,11-14. Even using 3-D TEE, planimetry of the annular area underestimated the MDCT area by up to 9.6%, due most likely to the lower spatial resolution associated with 3-D TEE volumetric imaging15. Valve undersizing by TEE has been previously observed in surgical series that have compared TEE and intraoperative valve sizing23, and more recently by pre-operative16,17,20 and post-operative CT studies24. Indeed, previous studies have highlighted the potential advantages of CT-guided measurement of the aortic annulus, due to this modality’s superior appreciation of the oval shape of the annulus based on 3-D isotropic high resolution, and less inter- and intra-observer variability6,7,11.
In our study, the measured sagittal diameter of the aortic annulus by TEE approximated to the short-axis diameter measured using CT, but was grossly undersized compared to the CT long-axis diameter. Thus, we used the mean diameter (mDiam-CT) for accurate valve sizing, as this value averages all diameters of the oval shape virtual annulus. Recently the area-derived diameter (mDiam-CT) has been described as the most reproducible MDCT measurement of the aortic valve annulus25.
Using this strategy, the proposed valve size for implantation was changed in 19.4% of cases, which led to a reduction in post-procedural AR ≥2, without an increased incidence of annulus rupture.
In the current study, a bicuspid aortic valve was observed more frequently in the CT-guided group compared to the TEE-guided group (8.6% vs. 0.6%, p<0.001). Explanations for this observation include the higher sensitivity of MSCT to detect bicuspid morphology, and an expansion of the indications for TAVI with increasing experience of this technology26.
Several studies have also demonstrated that cardiac magnetic resonance imaging (MRI) provides highly reproducible annulus measurements, which correlate with those achieved using MDCT27,28. This technique represents a promising modality for valve sizing in TAVI.
OPTIMAL VALVE SIZING
In this study, despite similar Diam-TEE in both groups, mDiam-CT was significantly larger than the Diam-TEE (23.6±2.0 mm vs. 22.3±1.9 mm, p<0.001) in the CT-guided group, and larger valve sizes were used (25.8±2.1 mm vs. 25.0±1.9 mm, p=0.001). The only predictor of post-procedural AR ≥2 was the ratio: valve size/mDiam-CT. The risk of annulus rupture was low (about 1%) and not increased in the CT-guided group despite implantation of larger valve sizes.
PREDICTOR OF POST-PROCEDURAL AORTIC REGURGITATION
In the current study, the only independent predictor of post-procedural AR ≥2 was the valve/mDiam-CT ratio (HR: 0.357, 95% CI: 0.166-0.768, p=0.008). While a previous study found a correlation between an index derived From TEE (the prosthesis/annulus discongruence ratio and post-procedural AR29), this was not evaluated in respect of post-procedural AR. Although we observed a trend towards an increased incidence of post-procedural AR in patients with high calcification scores of the aortic valve and annulus, these indices were not identified as the independent predictors of AR ≥2 by multivariate analysis. This result is in contrast to a previous study that identified the aortic valve calcification score as an independent predictor of significant AR11. The reason for these disparate results is not readily apparent; however, our data suggests that the uneven distribution of aortic valve calcification may be a contributing factor in patients with post-procedural AR, and it will be the subject of a future investigation. The method used to quantify aortic annulus calcification could be, in part, responsible for this discrepancy.
STUDY LIMITATIONS
Our study reports a single-centre retrospective TAVI cohort of limited size. We opted to include patients who received both the Edwards valve and the CoreValve, as this mixed cohort is our real clinical experience.
Patient treatment bias is inherent in non-randomised observational studies, and could have affected the comparison of clinical outcomes between the TEE-guided and CT-guided groups. Furthermore, significant differences in baseline characteristics exist between the TEE-guided and CT-guided groups. However, clinical outcomes were not the focus of this study and it is unlikely that these variables contributed significantly to the differences observed in post-procedural AR between the two imaging strategies evaluated.
The use of 2-D TEE rather than 3-D TEE for valve sizing in the TEE-sizing group could be considered a limitation of this study; however, this reflects day-to-day clinical practice and is in keeping with recent guidelines published by the American Society of Echocardiography and the European Association of Echocardiography10. Unmeasured confounders of post-procedural AR, such as degree of aortic valve calcification in the TEE-guided group, could have influenced our results. Randomised studies are therefore required to confirm our results.
Further studies of larger patient populations and patient randomisation to CT-guided and TEE-guided strategies are required to confirm our results.
Conclusion
CT-guided valve sizing in TAVI provides accurate assessment of the aortic annulus, and results in larger valve size implantation compared to TEE-sizing. This strategy is associated with a significantly lower incidence of post-procedural AR without an increase in annulus rupture. The “valve size/mDiam-CT ratio”, not “valve size/mean Diam-TEE” is the only independent predictor of post-procedural AR ≥2. Routine application of CT-valve assessment may reduce this important complication and could thus improve long-term patient outcomes after TAVI.
Acknowledgements
The authors would like to thank Mrs Catherine Dupic for her assistance in the preparation of this manuscript.
Conflict of interest statement
M. Romano is proctor for transapical-TAVI for Edwards. T. Lefèvre is proctor for transfemoral-TAVI for Edwards, and is consultant for Symetis and Direct Flow. B. Chevalier is a consultant for Medtronic. The other authors have no conflicts of interest to declare.
References

