
Introduction
Aortic valve-in-valve (aVIV) procedures are advancing the management of failed bioprosthetic surgical heart valves (SHV)1,2. As opposed to transcatheter heart valve (THV) replacement for native aortic stenosis, selection of the transcatheter THV size for aVIV procedures is based on the SHV size and not on anatomical measurements. However, accurate SHV size information may not be available in medical records. While computed tomography (CT) may be used to derive dimensions of the SHV, it does have limitations3. The aim of this study was to establish reference data for CT dimensions across commonly used aortic stented SHV types and sizes in order to determine the manufacturer’s labelled size from a CT data set.
Material and methods
STUDY POPULATION
CT data sets of patients who underwent aVIV planning for failed SHV at St. Paul’s Hospital (Vancouver, BC, Canada) between 2013 and 2018 were included. We also obtained 25 specimens from the Cardiovascular Tissue Registry at the Centre for Heart Lung Innovation (University of British Columbia and St. Paul’s Hospital), to provide a more complete representation of commonly encountered SHV (ex vivo imaging). The manufacturer’s labelled SHV size was determined from medical records. The Research Ethics Board at the University of British Columbia/Providence Health Care approved this study.
See Supplementary Appendix 1 for CT data acquisition and reconstruction, CT image and statistical analysis.
CT image analysis was performed as follows. First, the reconstruction phase with the best image quality was identified. Next, using multiplane reformats, a plane transecting the basal ring was created. Measurements were performed by fitting a circular region of interest to the centre of the radiopaque scaffold, to yield area and diameter.
Results
Average patient age at the time of CT imaging was 72±13 years; 101 (69%) patients were male. Median time between the initial SHV implantation and time of CT was 9.0 years (interquartile range 4 years).
Derivation of the study cohort is shown in Figure 1. CT appearance and alignment of the region of interest for measurement of SHV size are illustrated in Figure 2, for 10 common valve types. Measurement results are listed in Table 1 and illustrated in Figure 2 and Figure 3. There was excellent correlation between the CT-derived SHV size and the manufacturer size for all SHV (Supplementary Appendix 2, Supplementary Figure 1, Supplementary Figure 2).
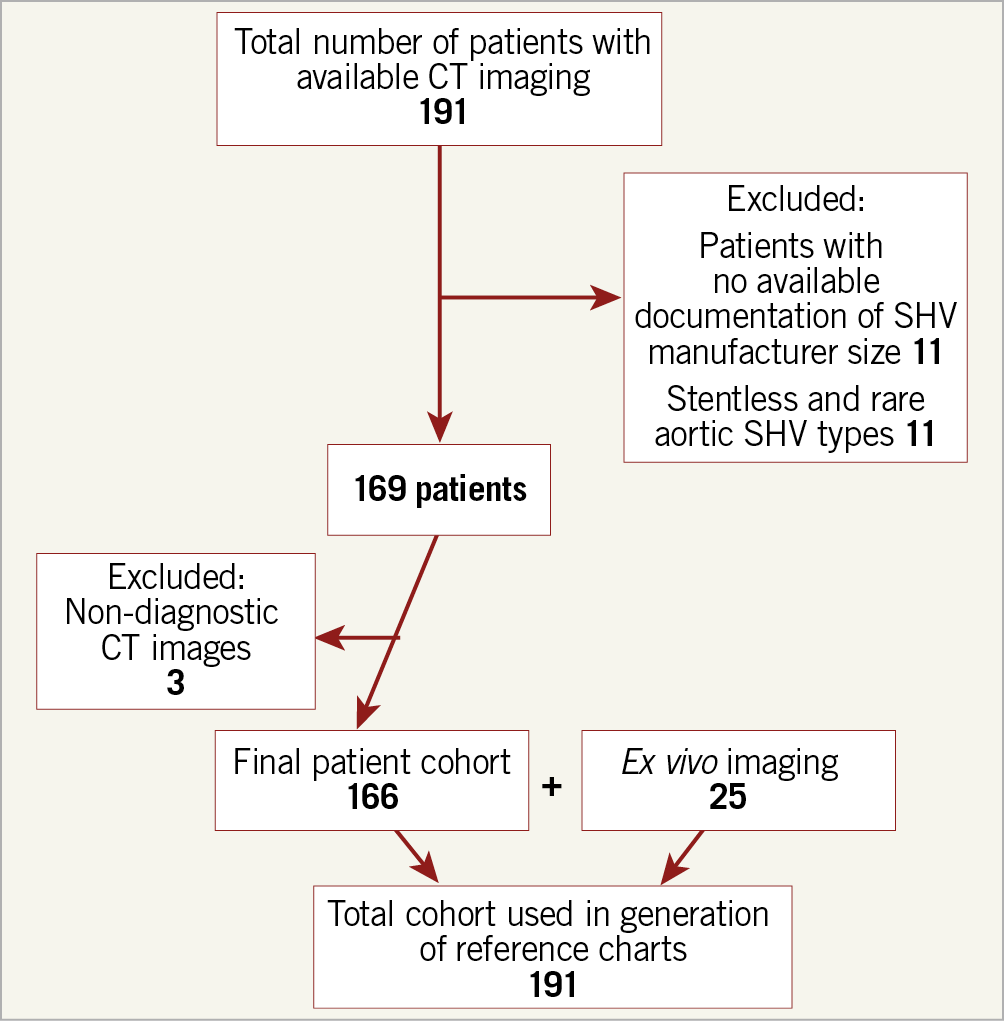
Figure 1. Flow diagram demonstrating patient inclusion and exclusion.
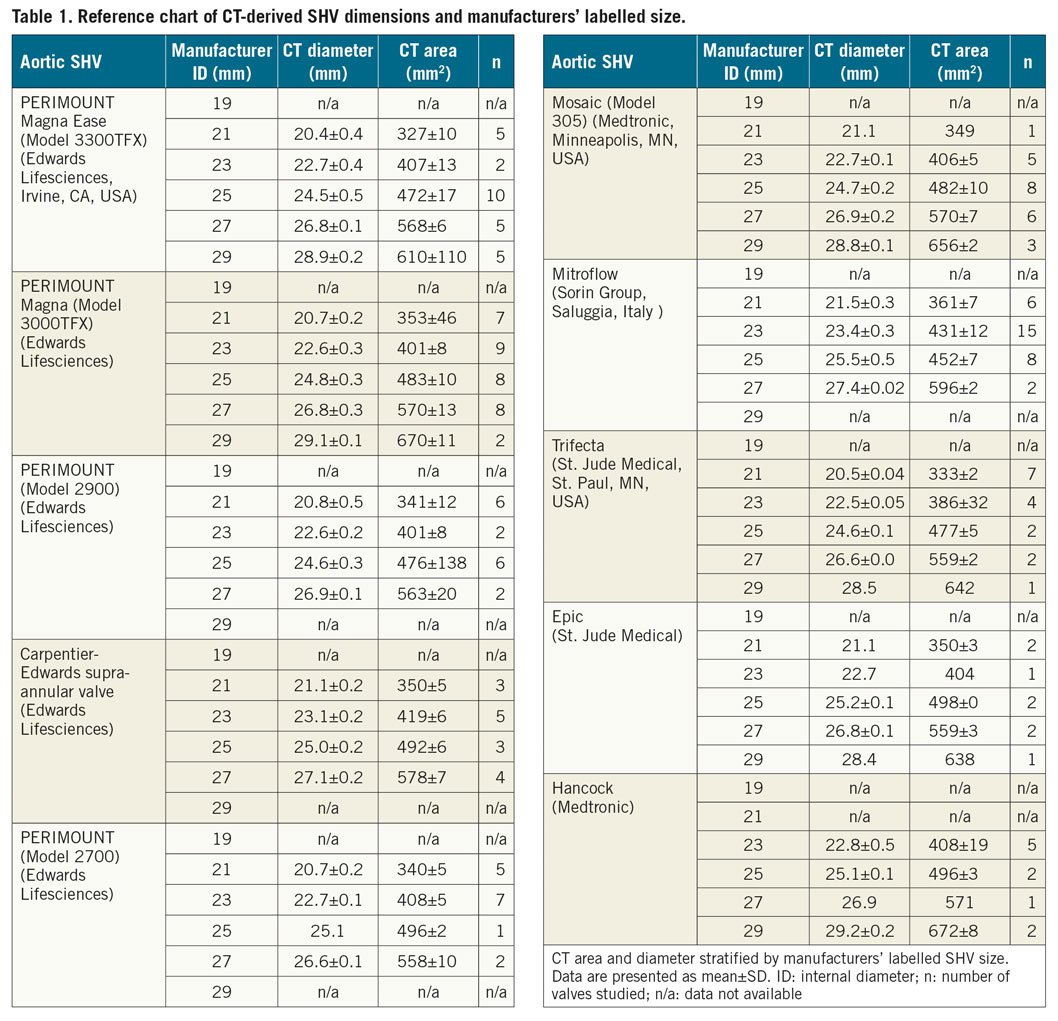
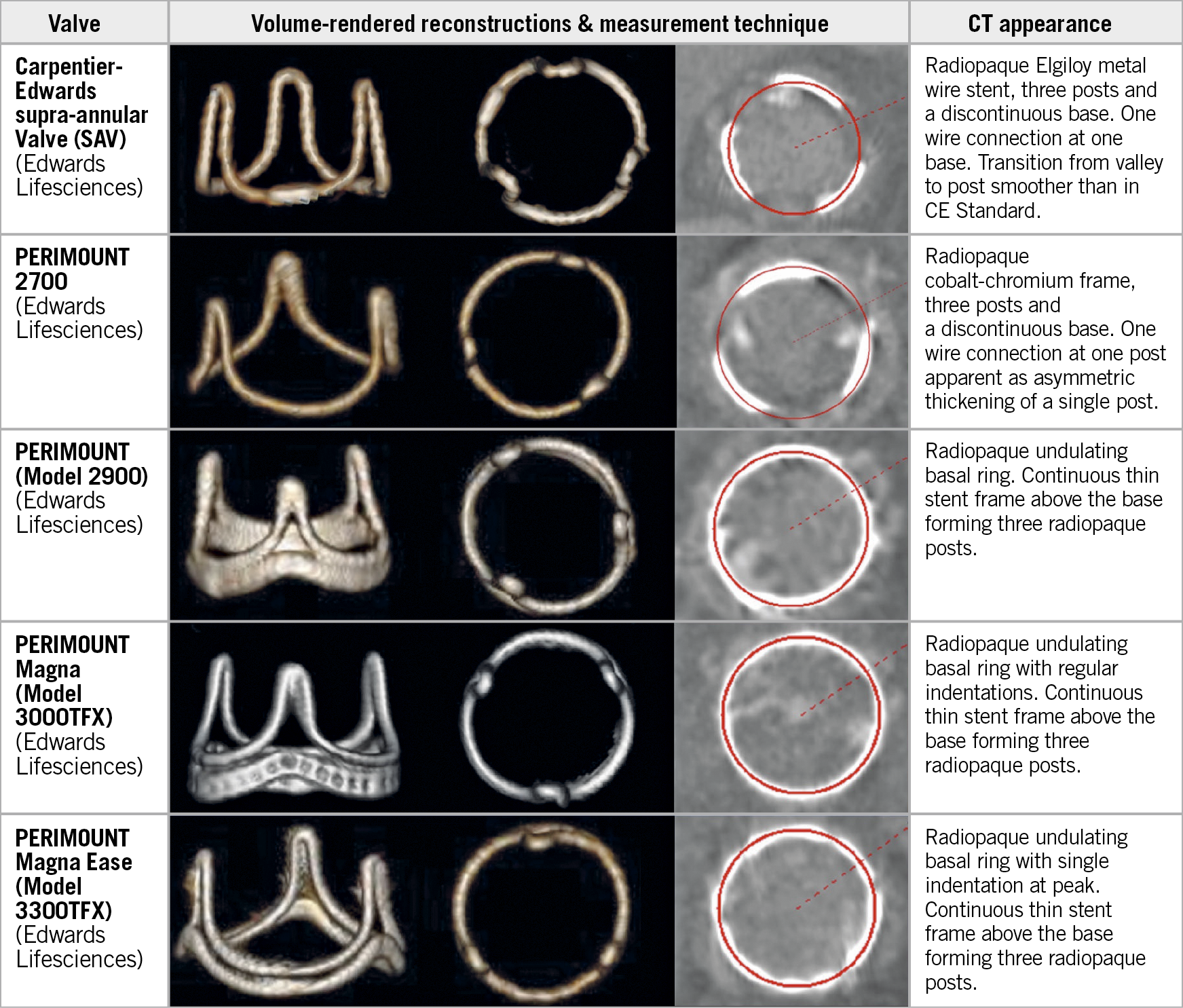
Figure 2. Lateral and en face CT volume-rendered images and multiplanar reformats aligned with the basal SHV ring, demonstrating circular ROI measurement (red circle) centred within the radiopaque contour (bone window) for 5 commonly used SHVs.
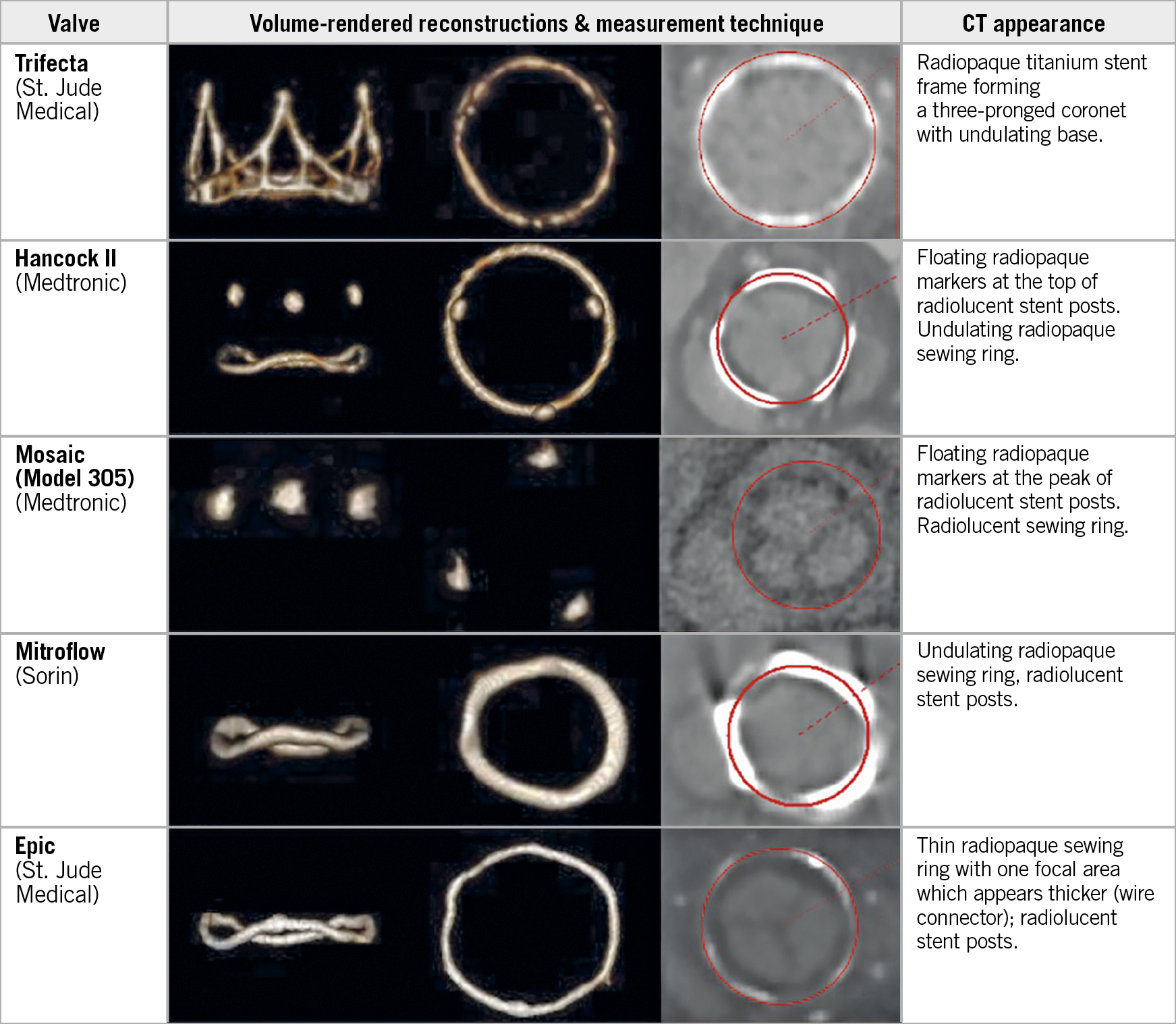
Figure 3. Lateral and en face CT volume-rendered images and multiplanar reformats aligned with the basal SHV ring, demonstrating circular ROI measurement (red circle) centred within the radiopaque contour (bone window) for 5 commonly used SHVs. Mosaic and Epic: measurement performed by fitting a circular ROI to the centre of the thin radiolucent sewing ring.
Discussion
There is increasing adoption of aVIV procedures for patients with failed surgical aortic bioprosthetic valves, given growing evidence that the procedure is safe and effective1,2. In planning for an aVIV procedure, CT may be used for measurement of the SHV size.
For planning an aVIV procedure, existing SHV size information is essential for determining THV size4. Lack of SHV sizes in aVIV procedures can lead to incorrect THV size selection, resulting in either undersizing and paravalvular leak or device embolisation, or oversizing leading to incomplete THV expansion and high transprosthetic gradients1. Patient SHV size documentation may be absent and thus determining SHV size from CT seems desirable, with CT imaging already required for aVIV planning5.
CT-based in vivo SHV sizing data are limited to a single study which evaluated SHV by measuring the inner contour of the basal sewing ring6. Importantly, the present study provides a more complete collection of valve types and sizes, and the measurement technique allows more robust translation to CT systems with different acquisition and reconstruction settings. We deliberately assessed CT dimensions along the centre of the radiopaque basal frame to reduce the impact of acquisition and reconstruction technique as well as artefacts.
CT-based SHV dimension assessment can be affected by artefacts due to the radiopaque component of the basal frame and sewing ring. These artefacts include blooming artefacts due to partial volume averaging as well as beam-hardening and streak artefacts. Blooming artefacts lead to overestimation of the metal component size, and thus to an underestimation when attempting to derive an internal diameter (ID) of the SHV from CT. Similarly, streak artefacts can impair accurate contour detection. Further, the extent of blooming and streak artefacts is influenced by tube potential, reconstruction technique and kernel, and window settings, as explained in Supplementary Appendix 3,7. Given that these factors influence the appearance of metal components, assessment of an ID appears less robust. Instead, the technique employed in this study is independent of the above factors, by measuring along the centre of the visualised radiopaque basal frame.
Measurement variability within individual valve type and size was limited. Only the Mitroflow SHV (Sorin Group, Saluggia, Italy) demonstrated higher measurement variability, due to the non-planar configuration of the basal ring. There was no overlap among assessed dimensions between different labelled valve sizes, permitting unambiguous determination of the labelled SHV size.
There is systematic discrepancy between the stent ID, defined as the inner diameter of the stent frame when covered with fabric or pericardium but without the valve leaflets, and the true ID, accounting for the valve leaflet insertions4. Importantly, CT does not appear capable of assessing the stent ID given the abovementioned impact on assessing the inner frame contour, nor can CT assess the true ID, which can only be assessed on a bench top. Thus, in the authors’ opinion, CT assessment should include a reproducible SHV size measurement, with subsequent comparison to a reference chart for determining the manufacturer’s labelled valve size, which can then be used to ascertain the stent ID and true ID, and the appropriate THV size using existing resources8.
Study limitations
This is a single-centre study, with the available valve types and sizes limited to local practice. Older-generation SHVs not commonly encountered in current clinical practice, such as the Carpentier-Edwards standard were not included9. The mechanism of aortic SHV degeneration and presence of pannus were not taken into account in the CT measurement.
Conclusion
The study provides a comprehensive reference chart of CT-derived SHV dimensions to allow identification of the manufacturers’ labelled size from CT measurements, and facilitate THV sizing for aVIV procedures.
|
Impact on daily practice CT may be used to determine the manufacturers’ labelled SHV size and guide aVIV procedural planning. |
Acknowledgements
The authors wish to acknowledge the support provided by Drs Bruce McManus and Paul Hanson in the Cardiovascular Tissue Registry at St. Paul’s Hospital.
Conflict of interest statement
J. Leipsic serves as a consultant and has stock options in HeartFlow and Circle Cardiovascular Imaging, institutional core laboratory agreements with Edwards Lifesciences, Abbott and Medtronic and receives speaking fees from GE Healthcare. J. Webb is a consultant to, and has received research funding from, Edwards Lifesciences, Abbott Vascular, Boston Scientific and ViVitro Labs. D. Wood is a consultant to Edwards Lifesciences and receives grant support from Edwards Lifesciences, Abbott Vascular, AstraZeneca and Boston Scientific. J. Sathananthan has received speaking fees from and is a consultant to Edwards Lifesciences. P. Blanke is a consultant for Edwards Lifesciences and Circle Cardiovascular Imaging, and provides CT core lab services for Edwards Lifesciences, Medtronic, Neovasc, and Tendyne Holdings, for which he receives no direct compensation. V. Bapat is a consultant to Medtronic, Boston Scientific, Meril, 4C and Edwards Lifesciences. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Supplementary data
To read the full content of this article, please download the PDF.

