Abstract
Aims: The expansion of TAVI will involve an increase in the frequency of emergent or late cardiac surgery after THV implantation. This study was designed to investigate the anatomical feasibility of surgical cross-clamp and aortotomy after TAVI through a post-TAVI CT-scan assessment.
Methods and results: We retrospectively analysed 117 CTs acquired after TAVI procedures with high stent prostheses in three high-volume centres between October 2008 and May 2017. The mean distance observed between the innominate artery and the top of the transcatheter heart valve was 45±11 mm, being <30 mm in 8/117 (6.8%) patients and <20 mm in none. The mean distance between the sinotubular junction and the first free site for aortotomy was 22±7 mm (>20 mm in 78/117 [66.7%] cases). A total of 56/117 (47.9%) patients showed a complete continuous contact between the anterior aortic wall and the anterior part of the valve stent.
Conclusions: Aortic cross-clamp appears not to be an issue when cardiac surgery is needed after TAVI; however, a careful and possibly higher aortotomy may be required. CT should be performed prior to planned cardiac surgery after TAVI to determine a safe positioning for aortic cross-clamp and aortotomy.
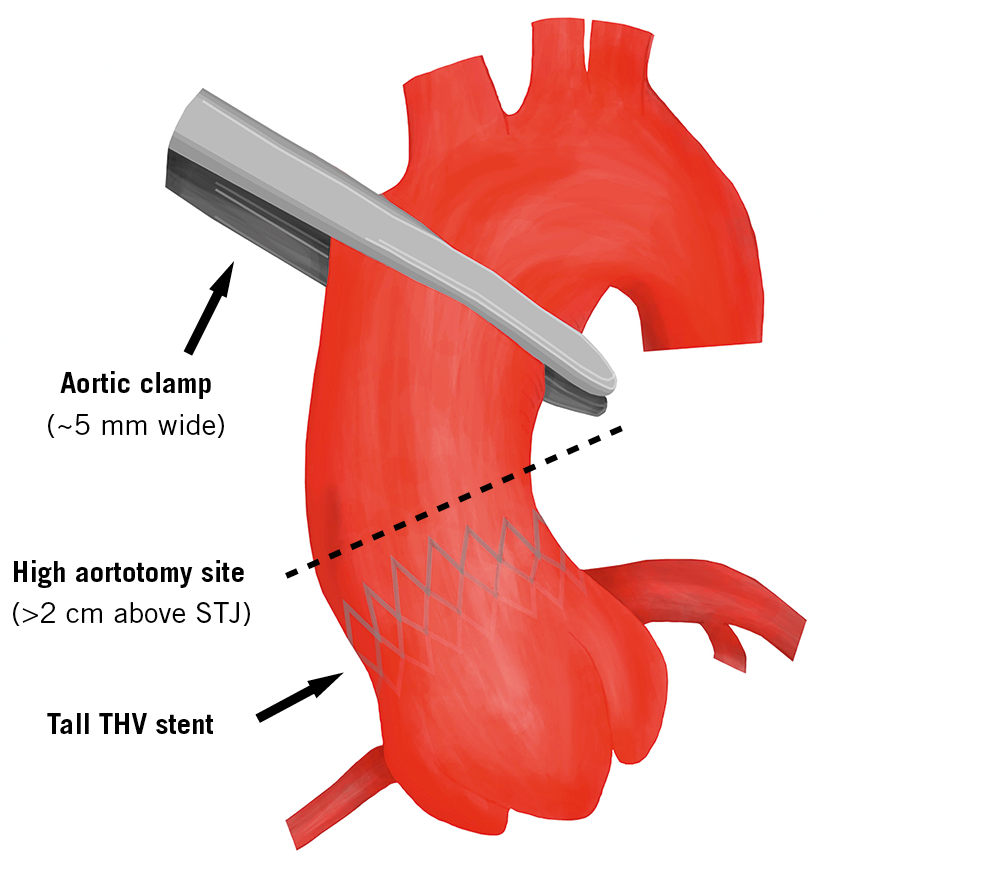
Visual summary. Proper aortic cross-clamp and careful higher aortotomy after TAVI.
Introduction
Transcatheter aortic valve implantation (TAVI) was clinically introduced first by Alan Cribier in 2002 and has since become the intervention of choice for symptomatic, severe aortic stenosis in elderly patients at increased surgical risk1,2,3,4. Cardiac surgery after TAVI is rare in this population (<1%), and typically reserved for cases of life-threatening complications such as device embolisation, heart rupture, aortic dissection, and endocarditis5,6,7,8. In recent years, the landscape of TAVI has evolved to incorporate patients at younger age and lower surgical risk, which implies longer life expectancy9,10,11. As a consequence, the number of patients treated with TAVI who will require subsequent cardiac surgery, both acutely (due to the above-mentioned complications) and later on during follow-up (due to late endocarditis, valve degeneration and other indications), has expanded.
It has long been debated whether the presence of TAVI devices with tall stent frames in the ascending aorta may jeopardise the surgeon’s ability to clamp and open the aorta7. To date, only a few anecdotal cases of transcatheter heart valve (THV) explantation have been reported in the literature12,13,14,15,16,17; no specific data are available regarding the existence and incidence of this theoretical problem. The aim of this study was to assess the anatomical feasibility of surgical aortic cross-clamp and aortotomy after TAVI based on the analysis of post-TAVI computed tomographies (CT).
Materials and methods
PATIENTS
This study enrolled patients who underwent TAVI at three high-volume centres (San Raffaele University Hospital, Milan, Italy; Rigshospitalet, University of Copenhagen, Denmark; and Semmelweiss University, Budapest, Hungary) between October 2008 and May 2017.
A total of 117 CT scans acquired after TAVI and specifically cardiac-gated with inclusion of the aortic arch were retrospectively reviewed. Reasons for post-TAVI CT acquisition were as follows: participation in a clinical study (91.4%, n=107), residual aortic regurgitation and evaluation for TAVI-in-TAVI (6.0%, n=7), TAVI complication assessment (ventricular septum defect 1.7%, n=2, left ventricular outflow tract obstruction 0.9%, n=1). Median time between the TAVI procedure and the CT scan was 451 (IQR: 290-780) days.
CT-SCAN ANALYSIS
Each centre provided multi-detector CT scans by means of DICOM files. Cardiac CT acquisitions were performed according to site-specific institutional protocols, comprising contrast-enhanced, multiphasic, gated imaging. San Raffaele Scientific Institute, Milan, Italy, served as the core lab for all CT analyses. Measurements were determined on orthogonal multi-planar reconstruction (MPR). A subgroup analysis showed no statistical difference when additional curved-MPR measure assessment was performed.
CT scans were analysed with the OsiriX DICOM Viewer MD 5.8 (Pixmeo SARL, Bernex, Switzerland).
Four principal measurements were evaluated (Table 1): 1) the shortest distance between the THV and the most inferior point of the innominate artery (Figure 1, green A); 2) the shortest distance between the centre of the virtual basal ring (VBR) and the most inferior point of the innominate artery ostium (Figure 1, green B); 3) the minimal distance between the sinotubular junction (STJ) and the first THV-free anterior aortic wall point (Figure 2, green C); and 4) the maximum gap between the anterior ascending aortic wall and the upper margin of the THV stent frame (Figure 2, green D). The aim of analysing these specific measures relies mostly on two aspects, for A+B and C+D distances, respectively: first, of having enough comfort space for the positioning of the surgical ascending aortic clamp, avoiding the innominate artery ostium and the THV stent below the aortic wall; and second, of performing a safe aortotomy by scalpel without the risk of becoming entangled with the TAVI upper stent.
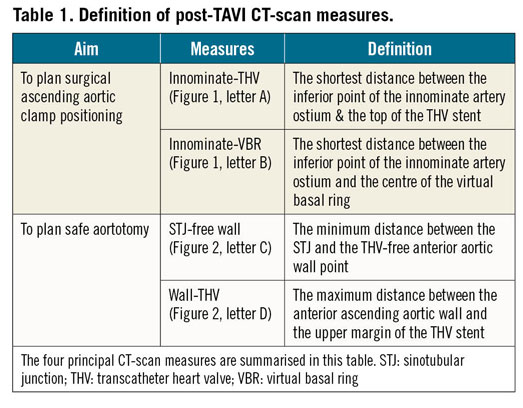
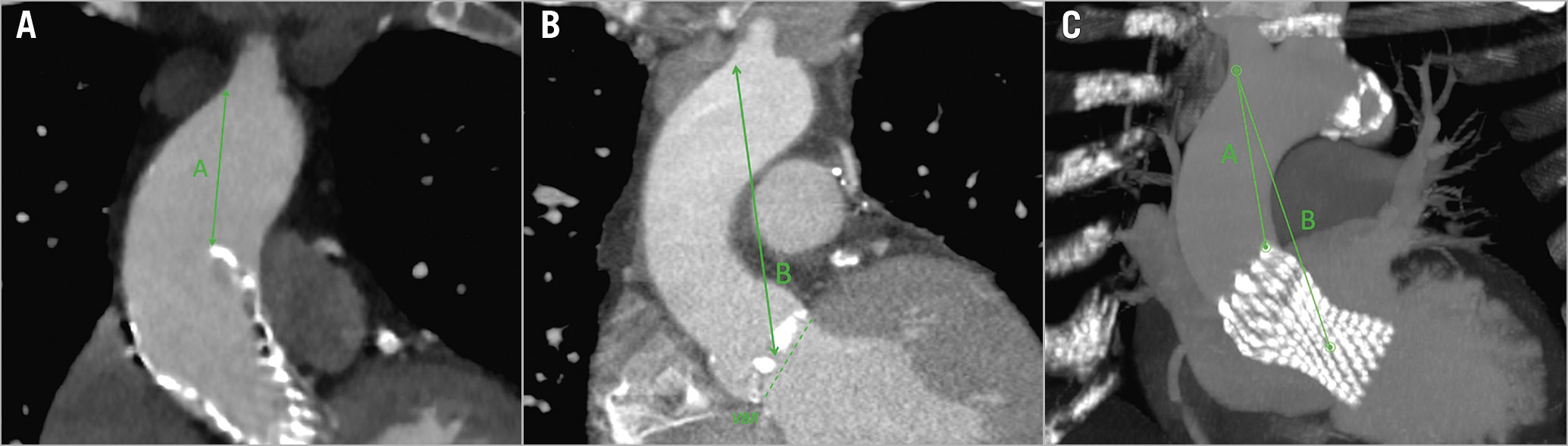
Figure 1. CT-scan reconstruction showing measures for aortic cross-clamping. Post-TAVI CT-scan reconstruction, showing the measures for the correct positioning of the aortic clamp, both in a 2D image (A, on the left, post-TAVI acquisition; B, in the middle, pre-TAVI acquisition) and in 3D MIP reconstruction (C, on the right): the shortest distance between the distal part of the THV stent and the inferior point of the innominate artery ostium (green A); and the shortest distance between the centre of the VBR and the inferior point of the innominate artery ostium (green B). 2D: two-dimensional; 3D: three-dimensional; CT: computed tomography; MIP: maximum intensity projection; TAVI: transcatheter aortic valve implantation; THV: transcatheter heart valve; VBR: virtual basal ring
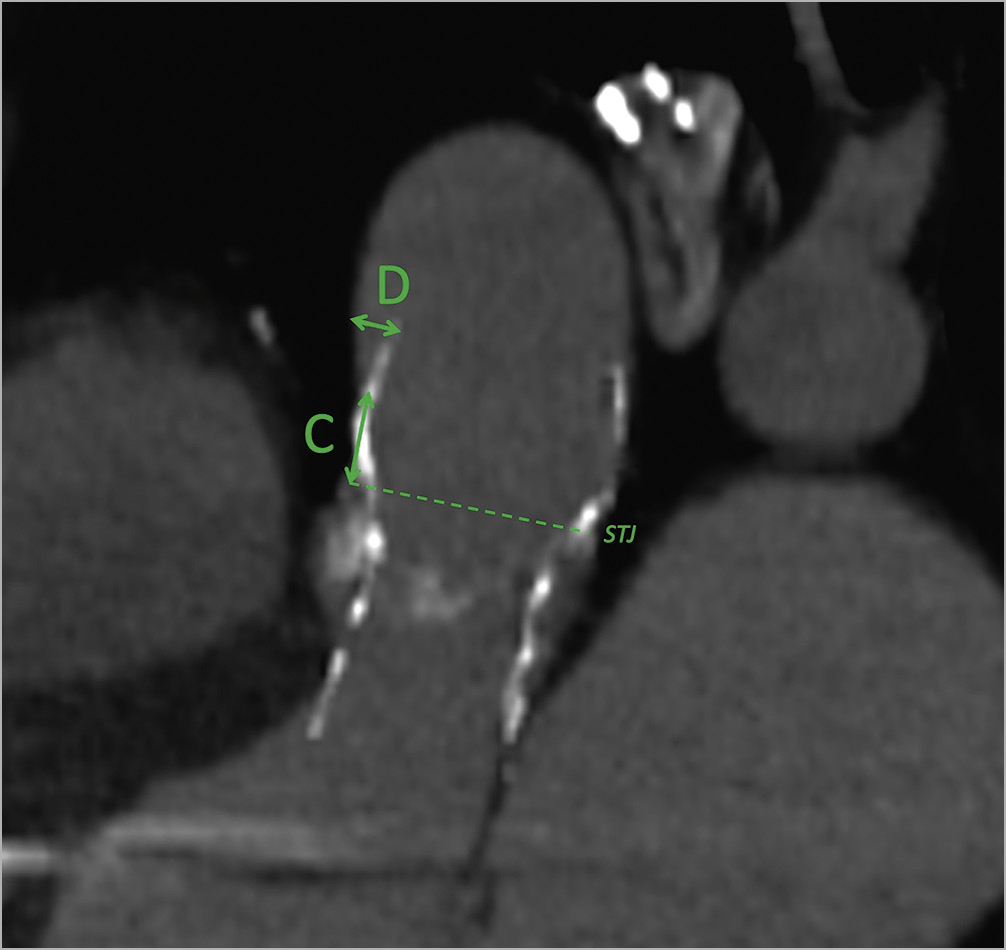
Figure 2. CT-scan reconstruction showing measures for aortotomy. Post-TAVI CT-scan reconstruction, oblique-sagittal view (sternum on the left of the image), showing the measures for an adequate aortotomy: the minimal distance between the STJ and the THV-free anterior aortic wall point (green C); and the maximum gap between the anterior ascending aortic wall and the upper margin of the THV stent (green D). CT: computed tomography; STJ: sinotubular junction; TAVI: transcatheter aortic valve implantation; THV: transcatheter heart valve
STATISTICAL ANALYSIS
Data from each centre were collected in a dedicated database and statistical analysis was conducted at the San Raffaele Scientific Institute. Categorical variables were expressed as absolute and relative frequencies (%). Continuous variables were reported as mean±SD or median and (Q1–Q3) for normally and not normally distributed data, respectively. For continuous variables, the normality of distribution was assessed with the Kolmogorov-Smirnov test. Subgroup analysis was performed using the ANOVA Kruskal-Wallis test. All statistical analyses were performed using SPSS, Version 22 (IBM Corp., Armonk, NY, USA).
Results
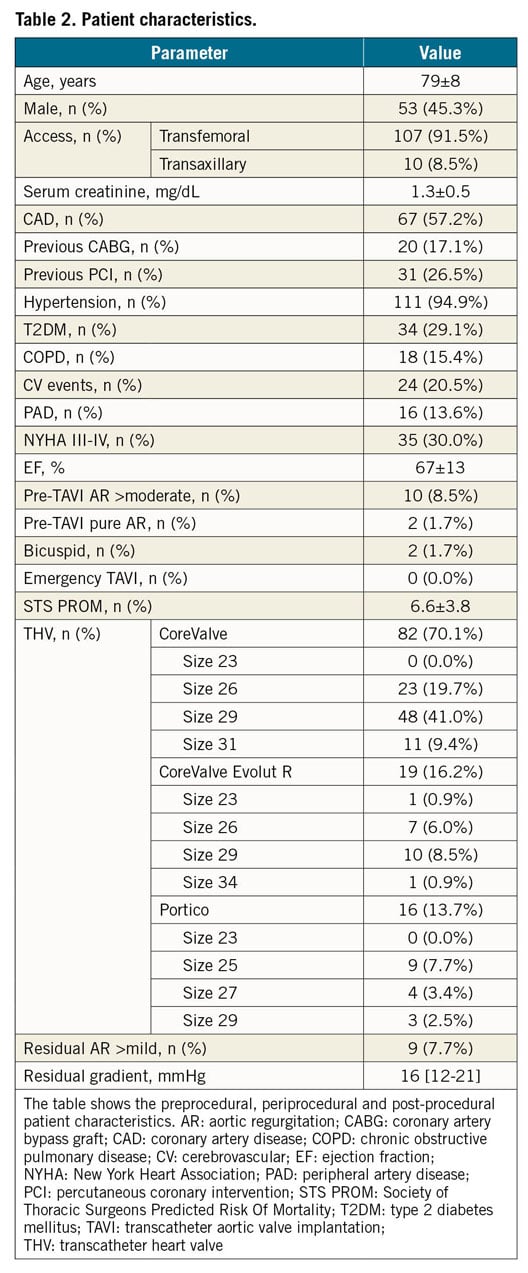
The THV used were: CoreValve n=82 (size 26, n=23; size 29, n=48; size 31, n=11); CoreValve Evolut R n=19 (size 23, n=1; size 26, n=7; size 29, n=10; size 34, n=1); Portico n=16 (size 25, n=9; size 27, n=4; size 29, n=3). Details of patient characteristics are provided in Table 2. As per the study design, only TAVI devices with a high stent frame were assessed (CoreValve® and CoreValve® Evolut™ R [Medtronic, Santa Rosa, CA, USA]; Portico™ [Abbott Vascular, Santa Clara, CA, USA]). The ACURATE neo™ device (Boston Scientific [formerly Symetis], Marlborough, MA, USA) is different from other circular tubular stents or self-expanding valves as it has only three stabilisation arches in the ascending aorta. To limit the introduction of possible confounding factors, we decided not to include the ACURATE neo in our study.
The ethics committee approved this study and waived individual consent for the retrospective analysis.
The mean observed distance between the VBR and the innominate artery ostium was 89±10 mm (Table 3). The CT scans revealed that no patient had a distance <20 mm between the THV and the innominate artery, with the shortest observed measurement being 22 mm in two cases. Eight patients had a THV to innominate artery distance <30 mm (25 mm in 4 cases, 24 mm in 1, 23 mm in 1, and 22 mm in 2).
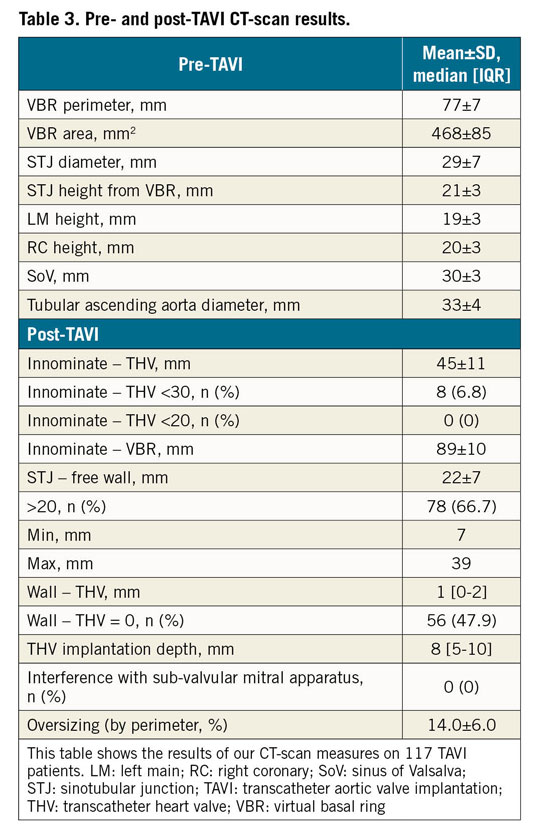
The mean distance between the STJ and the first possible site for aortotomy free from the underlying THV stent frame was 22±7 mm, being >20 mm in 78/117 (66.7%) cases (minimum 7 mm, maximum 39 mm). The anterior aortic wall came into contact with the stent frame in all cases. In 47.9% of cases there was complete continuous contact between the anterior aortic wall above the STJ and the anterior part of the THV stent frame. More than half of the patients (61/117 cases [52.1%]) showed a separation between the THV upper crown and the anterior aortic wall (mean distance 1 [0-2] mm, maximum distance 5.1 mm in two cases) (Figure 3).
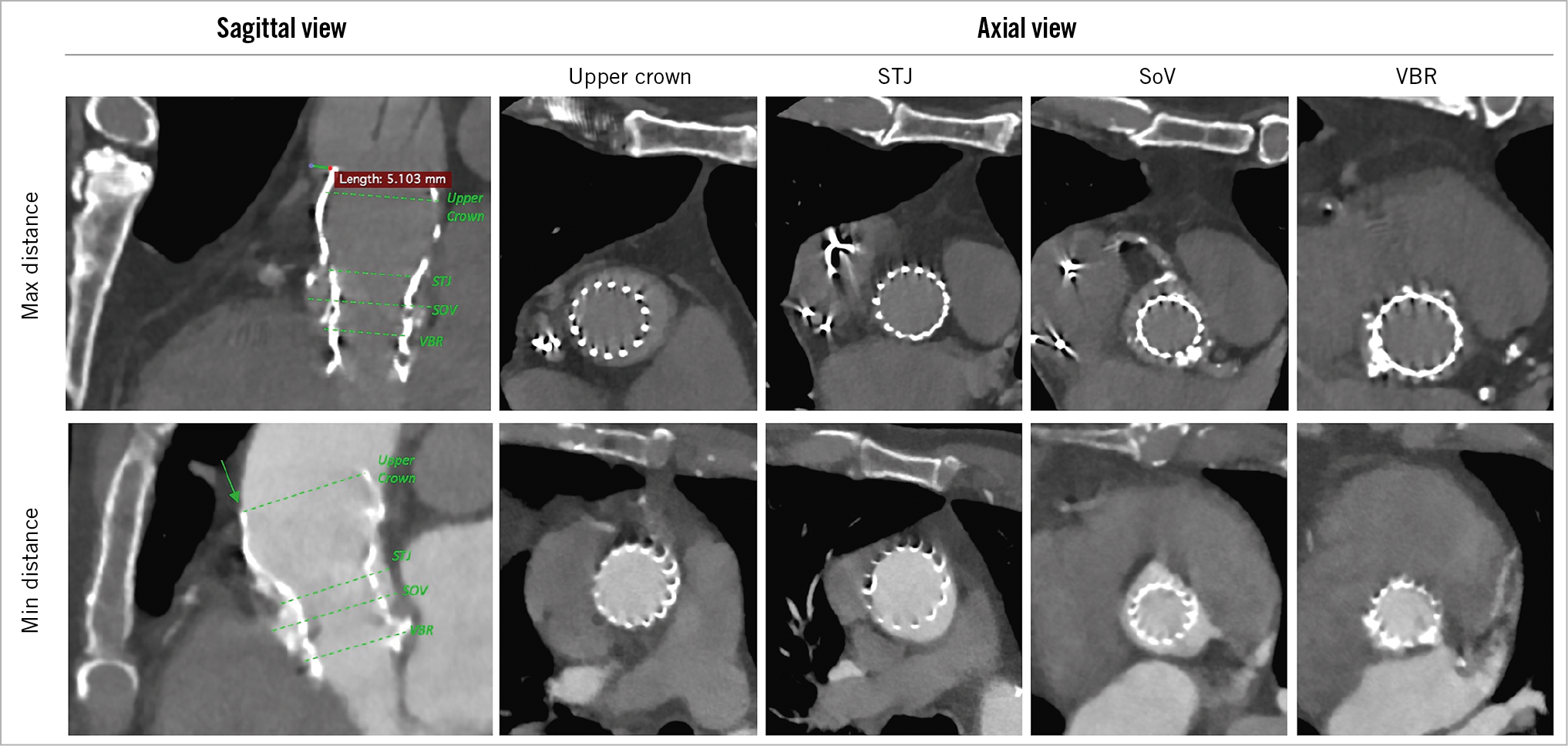
Figure 3. CT-scan multi-planar reconstruction: comparison between maximum and minimum anterior aortic wall-THV distance. On the left, oblique-sagittal views with a maximum gap of 5.1 mm (upper left) and complete aortic-THV contact (lower left). On the right, axial scans of the self-expanding prosthesis at different aortic levels: THV upper crown in the tubular tract of ascending aorta; at the STJ; at the SoV; at the VBR. The arrow indicates the complete contact between the THV upper cage and the anterior ascending aortic wall. CT: computed tomography; SoV: sinus of Valsalva; STJ: sinotubular junction; THV: transcatheter heart valve; VBR: virtual basal ring
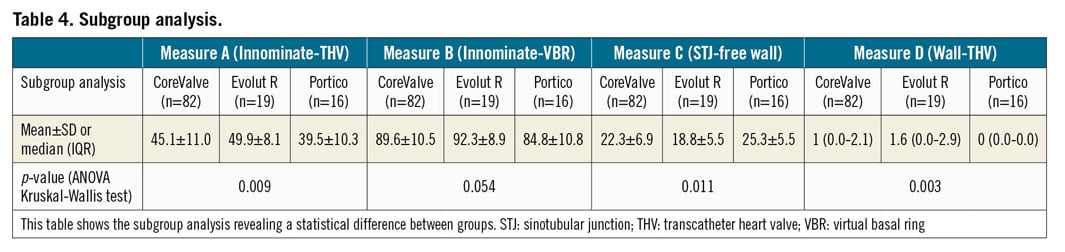
As reported in Table 4, subgroup analysis showed significant differences among the CoreValve, Evolut R and Portico groups, except for measure B: compared to the others, patients who had a Portico prosthesis implanted revealed relatively less space for aortic clamp positioning (39.5±10.3 mm; p=0.009) and close contact between the aortic wall and the THV upper crown (0 [0.0-0.0] mm; p=0.003).
Discussion
Over the past decade, a few THV explantation procedures have been described in the literature. There has been debate within the surgical community regarding whether the presence of a cumbersome metallic crown in the ascending aorta may affect aortic cross-clamping and aortotomy, thus increasing the risk and technical complexity of open heart surgery after TAVI12,13,14,15,16,17,18.
To the best of our knowledge, this is the first multicentric study to analyse systemically the anatomical feasibility of ascending aorta surgical manipulation after TAVI, based on post-TAVI CT scans of more than one hundred patients. The main findings of this study were twofold: 1) no patient showed a distance <20 mm between the distal part of the THV stent frame and the innominate artery; 2) the mean distance between the STJ and the first possible site for aortotomy was 22±7 mm.
Although preliminary, the results of our study are reassuring. Surgical ascending aortic clamps are 0.5±0.1 cm wide and the clamp positioning on the aorta takes no more than 2 cm (Figure 4). In our series, with approximately 9 cm from the VBR to the innominate artery, only eight patients (6.8%) presented a distance of <30 mm between the THV stent frame and the innominate artery and none of the patients had a distance of <2 cm. Our data suggest that the presence of a THV stent across the site of an aortic clamp is likely to be a rare occurrence. We do acknowledge, however, that the quality of the aortic wall may affect the quantity of tissue required for a safe manoeuvre, depending on factors beyond the distance from the THV, such as elasticity, stiffness and calcifications.
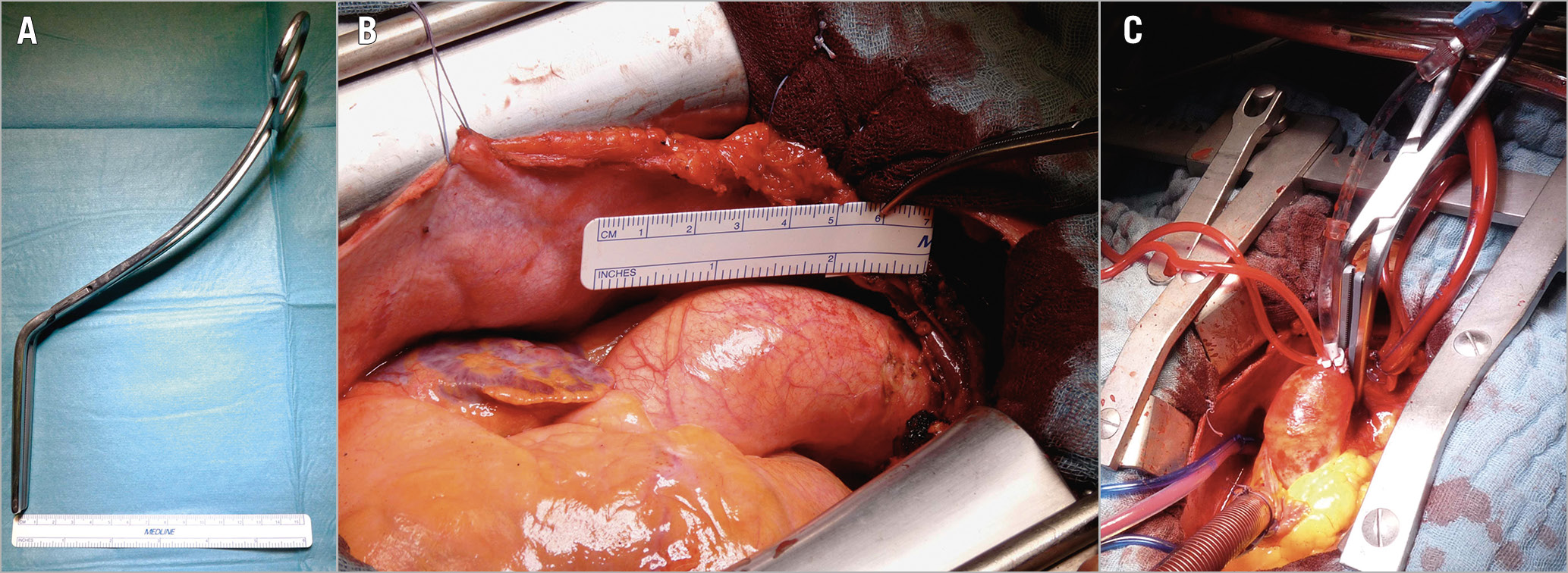
Figure 4. Aortic cross-clamping. A) The aortic clamp width is about 1 cm. B) Surgical view of the ascending aorta. C) Surgical aortic cross-clamping.
Opening of the ascending aorta after TAVI, however, may prove to be more complicated. The surgical site for aortic incision during an aortic valve replacement is generally 1 cm above the STJ in the proximity of the so-called “plica transversae aortae” or “fold of Rindfleisch”. In our study, almost half (47.9%) of the patients examined had extensive contact between the TAVI stent and the anterior aortic wall from the STJ upwards. The first site free from the TAVI stent eligible for easy cutting was approximately 2.2 cm distal from the STJ and no less than 0.7 cm. Given these findings, a high first incision for aortotomy in the presence of a TAVI device is advisable (Figure 5, Moving image 1, Moving image 2). In addition, considering the difficulty of THV detachment in the aortic root in case of strong adhesions, a longitudinal instead of transverse aortotomy could be considered, maybe with extension towards the aortic annulus.
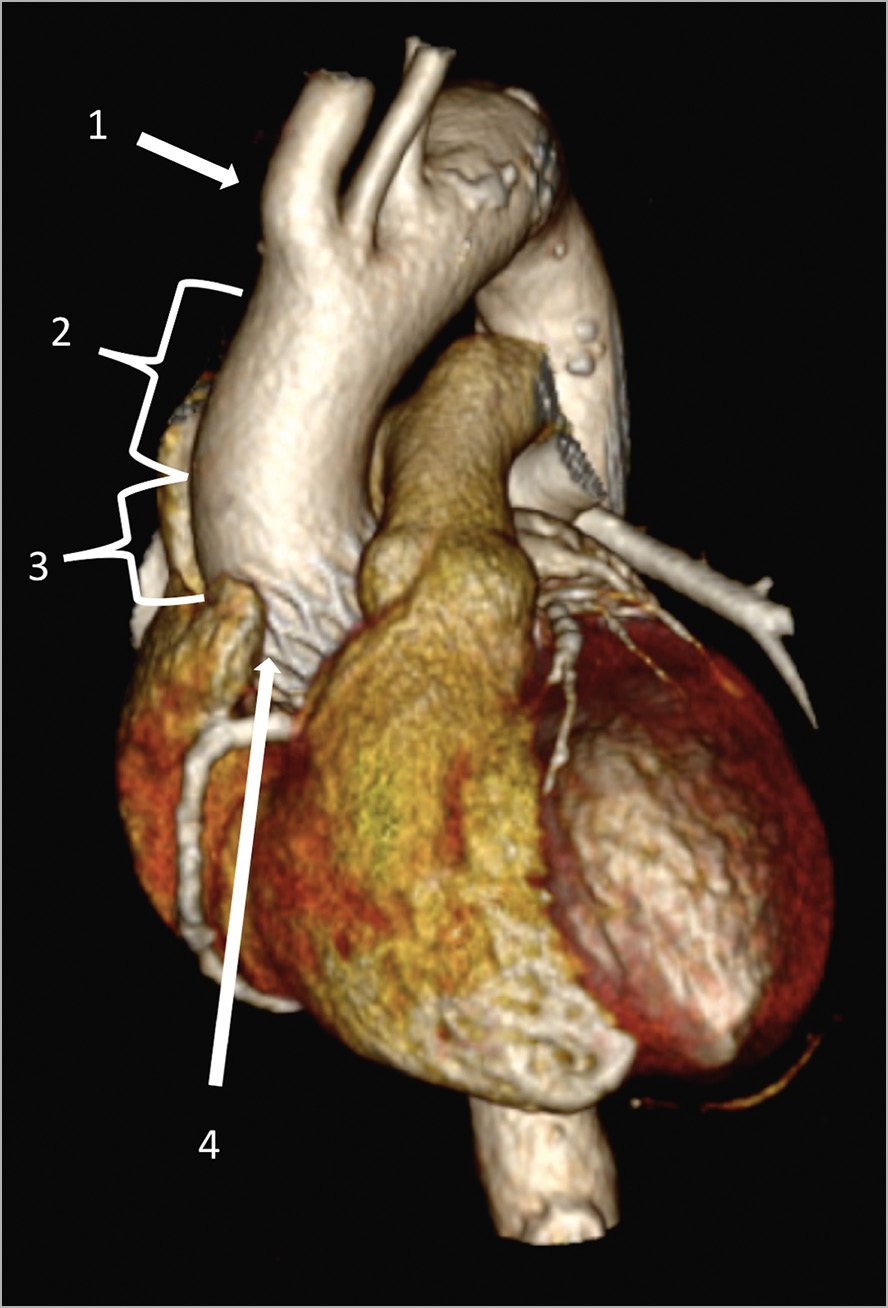
Figure 5. Three-dimensional cardiac CT-scan reconstruction. Site for an adequate aortic cross-clamping (2) and aortotomy (3) between the ostium of the innominate artery (1) and the upper crown of the THV stent (4). CT: computed tomography; THV: transcatheter heart valve
It must be underlined that two different scenarios of surgery after TAVI exist - acute emergent surgery and non-emergent follow-up surgery. Only rarely does emergent surgery need to be performed after TAVI, typically to solve a life-threatening complication. From 2013 to 2016, the European Registry on Emergent Cardiac Surgery during TAVI (EuRECS-TAVI) reported a rate of 0.76% (211/27,760) for emergency cardiac surgical procedures after transfemoral TAVI7. Emergency surgery after TAVI represents a technically demanding scenario in which fast and effective time management is important. The complexity of the situation combined with patients’ pre-existing comorbidities may explain the poor outcomes currently associated with emergent surgery after TAVI11. While the number of TAVI procedures continues to grow steadily annually, it is possible that concurrent advances in medical technology, technique, and knowledge will prevent a simultaneous increase in the proportion of emergency cardiac surgeries following TAVI.
As younger and lower-risk patients undergo TAVI, the number of patients who may require open heart surgery months or years following THV placement will increase12,13,14,15,16,17,18. This poses a challenge for surgeons, given the likelihood that stent endothelialisation will develop as time passes from the index procedure. Although data on this issue are currently sparse, some initial experiences have been described. In 2017, van Kesteren et al reported an autopsy series of 72 TAVI patients showing THV stents with intimal tissue overgrowth just six weeks after implantation19. In a more specific analysis, Noble et al performed four autopsies on patients who died between 3 and 350 days after CoreValve implantation. The histopathologic examination revealed different time points in the integration of the CoreValve stent into the aortic wall. During the first three months post THV implantation, the process begins with early fibrin deposits associated with a local inflammatory response. Eventually, late neointimal tissue develops covering the frame struts and completing the endothelialisation process of the implanted valve20. An extensive endothelial covering of the TAVI stent could create an unicum between the aorta and the THV, making it challenging for surgeons to perform a safe and effective aortotomy through the stent, sometimes increasing the risk of aortic dissection, thus requiring an ascending aorta or an entire aortic root replacement such as the Bentall procedure. In addition, the need for a high positioning of the aortic clamp will consequently require a different site for aortic cannulation: the usual placement of the arterial cannula in ascending aorta may not be possible and the cannulation of the aortic arch, axillary or femoral artery could be required.
Even if some reported cases of THV explantation have shown a relatively simple procedure of endarterectomy and THV detachment in expert surgical hands12,13,14,15,16,17, THV removal in such a scenario may be technically difficult without damaging the aortic wall itself, and a CT scan could help in the planning of the entire procedure.
During our imaging analysis, we noticed several different patterns of THV positioning inside the aorta, especially for the devices with the longest stents. According to the natural shape and curvature of the aorta, it was observed that almost all THV devices were less in contact with the posterior-left lateral aortic wall. Therefore, in cases of full contact between the THV and the anterior aortic wall, the left side of the aorta may be a good initial spot to start the incision and from where to proceed with aorta-THV detachment. In a similar way, no cases of direct contact between the THV stent and the aortic root wall were observed at the level of the sinus of Valsalva (SoV). In fact, at that plane, the middle part of the CoreValve and Portico prostheses becomes thinner and may represent an easier site for explantation compared to the upper crown, larger and in contact with the vessel endothelium, which is generally the first site where the surgeon begins the stent detachment from the aortic wall, just below the aortotomy. However, for an isolated surgical aortic valve replacement (SAVR), the aortotomy site is commonly in the ascending aorta and not in the aortic root. This means that the first part of the explantation procedure may represent the most difficult one, becoming safer later, during the removal of the lower part of the stent at the SoV and annular plane.
In subgroup analysis, Portico prostheses showed slightly but statistically significantly higher implants, resulting in a reduced aortic clamping zone and continuous contact between the aortic endothelium and the THV stent, thus eventually making aortic manipulation more challenging for the surgeon.
Based on the results of the present study, a CT scan appears to be able to provide useful information prior to cardiac surgery after TAVI. Even if contrast medium acquisitions provide more precise measurements, a low-dose contrast injection or even no contrast CT scans could be sufficient for the surgeon to plan the procedure21.
Study limitations
The retrospective nature and relatively small sample size are the main limitations of this study. Furthermore, this was a theoretical analysis performed on CT-scan data: no cases of cardiac surgery following TAVI were reported in this series, even if a few have been observed in our institutions. Large numbers from direct surgical operations will be required to confirm these findings.
Conclusions
Our results suggest that aortic cross-clamping appears to be unhindered by a THV when cardiac surgery is required after TAVI, but a careful and possibly higher aortotomy may be necessary. If possible, an extensive CT-scan analysis should be performed prior to cardiac surgery in patients previously treated with TAVI, with assessment of specific measures in order to plan a safe positioning of the aortic clamp and aortic incision.
Impact on daily practiceTAVI has become the first choice to treat severe aortic stenosis in elderly, high-risk patients. However, today’s TAVI population is progressively becoming lower-risk and younger and consequently the need for surgery after TAVI may increase. This is the first CT-scan study to investigate the theoretical anatomical feasibility of surgical ascending aortic manipulation after TAVI systematically. Our results show that aortic cross-clamping appears not to be an issue when cardiac surgery is needed after TAVI; however, a careful and possibly higher aortotomy may be required. Large numbers from direct surgical operations will be required to confirm our findings. More evidence on possible THV endothelialisation years after TAVI is needed.
|
Acknowledgements
We wish to acknowledge the Alfieri Heart Foundation for supporting data collection and analysis of this research.
Conflict of interest statement
O. De Backer has received consultant fees from Abbott and Boston Scientific. L. Søndergaard has received consultant fees and institutional research grants (not related to this publication) from Abbott, Boston Scientific, Edwards Lifesciences, Medtronic and Symetis. M. de Bonis is a consultant for Abbott Vascular and Medtronic. M. Montorfano serves as proctor for Edwards Lifesciences, Boston Scientific and Abbott Vascular. A. Latib is a consultant for Medtronic, Abbott Vascular and Edwards Lifesciences. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. 3D CT-scan reconstruction of measure A at minimum. Three-dimensional cardiac CT-scan reconstructions of the heart. Focusing on the ascending aorta, the THV (CoreValve) stent could be observed, as well as its relationship with the anterior aortic wall. We show the minimum distance (22 mm) observed between the innominate artery ostium and the THV upper cage (measure A).
Moving image 2. 3D CT-scan reconstruction of measure A at maximum. Measure A up to a maximum distance of 69 mm, which provides a more comfortable site for aortic cross-clamping.

