Abstract
Transcatheter aortic valve implantation (TAVI) has been an important breakthrough in the treatment of patients with symptomatic, severe aortic stenosis and contraindications for surgical aortic valve replacement. Accurate aortic root measurements and evaluation of spatial relationships with the coronary ostia are crucial in pre-operative TAVI assessment. In addition, characterisation of the peripheral artery anatomy and aorta is an important key step in the procedural feasibility evaluation. The present review article provides a practical approach, based on multimodality imaging, to select candidates for TAVI and to evaluate the procedural feasibility.
Introduction
Transcatheter aortic valve implantation (TAVI) has shown to be a feasible and effective therapeutic option for patients with symptomatic severe aortic stenosis and high operative risk.1 Since the first-in-man experience in 2002,2 several advances in TAVI techniques have led to improved success rate (94-97%) with acceptable procedure-related complications rate (5-18%).3-6 To date, two different devices have received the CE mark approval: the self-expandable CoreValve Revalving System (Medtronic, Minneapolis, MN, USA) and the balloon-expandable Edwards SAPIEN valve (Edwards Lifesciences, Irvine, CA, USA). The mid- and long-term results of this technique are encouraging and include improved New York Heart Association functional class and quality of life together with improved haemodynamic performance of the transcatheter aortic valve.7,8
However, in this emerging field, several issues remain source of debate. Vascular access, device sizing and positioning are the main challenges of this technique. In addition, vascular complications, incidence of stroke and electrical conduction abnormalities and long-term consequences of postprocedural aortic regurgitation (central or paravalvular leaks) still remain as major safety concerns.
Accurate multidisciplinary preprocedural evaluation of patients who are candidates for TAVI is mandatory to plan the most adequate procedural approach and to minimise the frequency of procedure-related complications.9 This preprocedural screening includes: confirmation of severity of degenerative aortic stenosis, evaluation of symptoms and analysis of the surgical risk and life-expectancy and assessment of the procedural feasibility and exclusion of contraindications for TAVI.9 Multimodality imaging, including 2- and 3-dimensional echocardiography, invasive angiography, multidetector row computed tomography (MDCT) and magnetic resonance imaging (MRI), plays a central role in the selection of candidates for TAVI, and is crucial to plan the TAVI technique. Echocardiography remains as the cornerstone technique to evaluate the anatomy and function of the aortic valve and to measure the aortic valve annulus and aortic root dimensions, a key step in the selection of the prosthesis size. However, there is a growing interest on the use of 3-dimensional imaging techniques (real-time 3-dimensional echocardiography, MDCT and MRI). These imaging techniques provide a more accurate assessment of the dimensions of the aortic valve annulus and aortic root.10-12 In addition, MDCT and MRI permit characterisation of the peripheral arteries and thoracic aorta, important steps to select the TAVI approach.13-15 Regardless of the imaging modality used, evaluation of patients who are candidates for TAVI should be performed by a highly experienced team on multimodality imaging in order to provide the most accurate sizing of the aortic valve annulus, aortic root and peripheral arteries.
The present document provides a practical approach, based on multimodality imaging, to select candidates for TAVI and to evaluate the procedural feasibility.
Evaluation of the aortic valve anatomy and morphology
Evaluation of the aortic valve anatomy is a crucial step in the evaluation of the patients who are candidates for TAVI.
According to current position statement, TAVI is indicated in patients with severe symptomatic aortic stenosis, high operative risk and tricuspid anatomy of the aortic valve.9 Bicuspid anatomy of the aortic valve is considered a contraindication for TAVI.9 However, highly experienced centres have also demonstrated the feasibility of this novel therapeutic technique in this particular valve anatomy.16 Echocardiography is the mainstay imaging technique to evaluate the anatomy of the aortic valve. In patients with poor acoustic windows, transoesophageal echocardiography is a suitable alternative to transthoracic echocardiography to define the aortic valve anatomy. In addition, echocardiography permits the assessment of the extent and location of aortic valve calcifications. The presence of severely calcified aortic valve with commissural fusion may challenge the deployment of the prosthesis whereas the presence of bulky calcifications may increase the risk of coronary ostium occlusion.17
The high spatial resolution of MDCT enables accurate visualisation of the aortic valve and accurate measurement of the anatomic valve area.18 The extent of valvular calcifications can be evaluated with this imaging technique providing exact characterisation of the location of the calcifications (commissures, free edge or base implantation of the leaflets) (Figure 1A). The specific location of valvular calcifications may determine the deployment of the transcatheter valve prosthesis and the presence of postprocedural aortic regurgitation.19 As indicated by Zegdi et al,19 the presence of heavily calcified aortic valve commissures may pose resistance to deployment of the prosthesis resulting in less circular deployed prosthesis and increased risk of gaps between the external surface of the prosthesis and the host native valve. The dimensions of these gaps will determine the severity of the paravalvular leak.19 In addition, the presence of bulky calcifications in the free edge of the leaflets may increase the risk of coronary ostium occlusion during expansion of the prosthesis.17 Recently, few studies have related the amount of aortic valve and aortic root calcifications as assessed with MDCT with the risk of significant postprocedural paravalvular aortic regurgitation.20 For both the CoreValve system and Edwards SAPIEN valve, the presence of heavily calcified native valves may challenge the deployment and positioning of the prosthetic valve resulting in less circular deployment of the valvular frame. As a consequence, there may be small gaps between the prosthetic frame and the native valve that result in paravalvular aortic regurgitation (Figure 1B).20,21
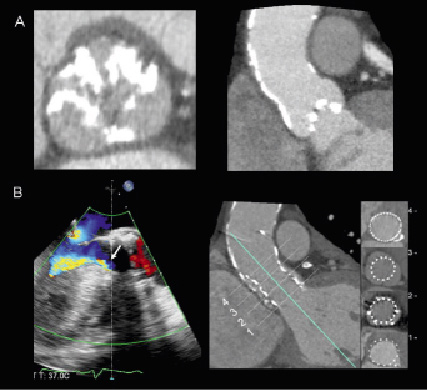
Figure 1. Aortic valve anatomy and extent and location of calcifications. MDCT provides accurate evaluation of the morphology and extent and location of the calcifications. The double oblique transversal plane through the aortic valve leaflets (Panel A, left) permits the visualisation of the leaflets and the number of commissures that will define the morphology of the valve (tricuspid/bicuspid). The distribution of the calcifications of the aortic root can be confirmed in the coronal or sagittal views (Panel A, right). The presence of extensive calcification of the aortic valve and aortic root may challenge the deployment and positioning of the transcatheter aortic valve (Panel B, right) and result in higher incidence of paravalvular regurgitation (Panel B, left, arrow). Adapted with permission from Schultz et al J Am Coll Cardiol 2009;54:911-918.
Measurement of the aortic valve annulus: key to select the prosthesis size
Accurate measurement of the aortic valve annulus is one of the most important steps in TAVI techniques. The selection of the prosthesis size relies on this measurement. Undersized aortic valve annulus may increase the risk of prosthesis migration or significant paravalvular leak. In contrast, oversized aortic valve annulus may increase the risk of rupture of this structure.9 The Edwards SAPIEN valve is available in two sizes: 23 mm for aortic valve annulus size between 18-22 mm and 26 mm for aortic valve annulus size between 21-25 mm.22 In addition, a 29 mm and 20 mm will be launched during this year (Edwards SAPIEN XT valve), providing a feasible alternative for those patients with too large or too small aortic valve annulus. The CoreValve Revalving System valve is available in 26 mm for aortic valve annulus size between 20-23 mm and 29 mm for aortic valve annulus size between 23-27 mm.22
Currently, there is no established gold standard imaging method to measure the aortic valve annulus. Transthoracic echocardiography is the most frequently used method. However, compared to transesophageal echocardiography, transthoracic echocardiography underestimates the aortic valve annular size by 1.36 mm, with a maximal difference of 4 mm between the two techniques.23 In contrast, 3-dimensional imaging techniques may provide the most accurate measurement of the aortic valve annulus.24,25 Unlike 2-dimensional echocardiography, 3-dimensional imaging techniques show the ellipsoid shape of the aortic valve annulus. Therefore, 3-dimensional imaging techniques permit the measurement of the planimetered annular area whereas 2-dimensional echocardiography assumes a circular annular area that may lead to important errors in the aortic valve annular size.24,25
Compared to 2-dimensional transesophageal echocardiography, Ng et al recently demonstrated the superior accuracy of 3-dimensional transesophageal echocardiography in the aortic valve annulus sizing.10 In 53 patients with symptomatic, severe aortic stenosis treated with TAVI, the accuracy of 2- and 3-dimensional transesophageal echocardiography in the measurement of the aortic valve annulus area was evaluated and compared to MDCT.10 Two-dimensional transesophageal echocardiography underestimated the planimetered aortic valve annular area by 16.4% whereas 3-dimensional transesophageal echocardiography underestimated the planimetered aortic valve annular area by 9.6% (Figure 2A).10
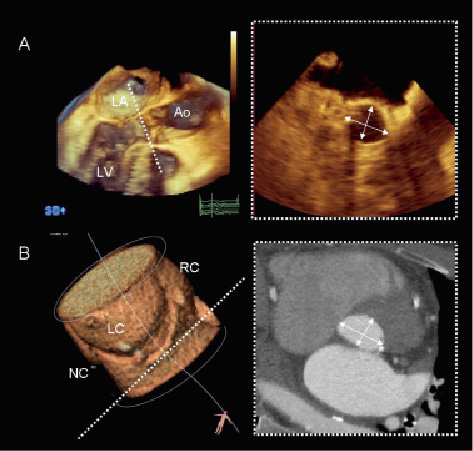
Figure 2. Assessment of the aortic valve annular size. Three-dimensional imaging techniques may provide the most accurate measurement of the aortic valve annulus. Three-dimensional transesophageal echocardiography (Panel A) and MDCT (Panel B) provide accurate orientation of the aortic valve annular plane by accurate alignment of the three orthogonal planes (3mensio Valves™, version 4.1; 3mensio Medical Imaging BV, Bilthoven, The Netherlands). Both techniques demonstrate the oval shape of the aortic valve annulus and provide two orthogonal diameters. Ao: aorta; LA: left atrium; LC: left coronary sinus; LV: left ventricle; NC: non-coronary sinus; RC: right coronary sinus
Current advances in MDCT and MRI have provided high-spatial resolution images that increase the accuracy of the aortic valve annular sizing. Particularly, MDCT has demonstrated to provide meaningful information on dimensions and shape of the aortic valve annulus, aortic root dimensions and spatial relationship of the aortic root and the surrounding structures.12,25,26 In 169 patients, the use of MDCT revealed the ellipsoid shape of the aortic valve annulus with the diameter of the coronal view larger than the diameter of the sagittal view (26.3±2.8 mm vs. 23.5±2.7 mm) (Figure 2B).12,25,26 These findings have important clinical implications on selection of TAVI strategy and, based on how the aortic valve annulus is sized, the selection of the prosthesis size may change significantly. 27,28 For example, Messika-Zeitoun et al evaluated 45 consecutive patients undergoing TAVI.27 Measurements of the aortic valve annulus diameters were performed with 2-dimensional transthoracic and transesophageal echocardiography and compared to MDCT. Despite good agreement in aortic valve annulus measurements between transthoracic and transesophageal echocardiography, transesophageal echocardiographic provided larger aortic valve annular dimensions and the decision to implant or to select the prosthesis size would have changed in 17% of the patients. In contrast, MDCT measurements showed a modest agreement with echocardiographic measurements and would have changed the TAVI strategy is a larger percentage of patients (38%).27 In addition, Schultz et al demonstrated that the mean diameter of the aortic valve annulus measured with MDCT (calculated as the mean between the minimum and the maximum diameter) had the best agreement with the prosthesis size selected by the operator (74% of the patients).25 In contrast, based on the minimum or the maximum diameter, the number of patients who would not qualify for TAVI due to too small or too large aortic valve annulus was 24% and 36%, respectively.25 Nevertheless, in both studies, selection of prosthesis size based on transesophageal echocardiographic measurements yielded good results with no significantly increased rate of complications (moderate or severe aortic regurgitation or device migration).25,27 Definition of the cut-off values of aortic valve annular dimensions to select the prosthesis size based on different currently available imaging modalities needs additional prospective studies.
Similarly to MDCT, MRI permits the assessment of the aortic valve annular size and shape. Burman et al confirmed in 120 consecutive patients with normal aortic valve anatomy and function the ellipsoid shape of the aortic valve annulus with the diameter on the coronal plane larger than the diameter on the sagittal plane (26.2±2.3 mm vs. 22.2±2.4 mm).29 Although the radiation exposure may be less important in elderly population candidates for TAVI, it may limit the use of MDCT in younger populations. Therefore, MRI constitutes a suitable imaging tool to accurately measure the aortic valve annulus in these populations.
Recently, the accuracy of 2-dimensional echocardiography and MDCT and MRI to measure the aortic valve annulus in patients who might be candidates for TAVI has been evaluated.11 Compared to perioperative measurements, MRI and MDCT provided the most accurate measurements. Therefore, the use of these 3-dimensional techniques in the preprocedural evaluation of patients who are candidates for TAVI may yield a more precise measurement of the aortic valve annular size, more precise selection of the prosthesis size and lower frequency of paravalvular leak.24 However, as indicated before, prospective studies are warranted to establish the gold standard method to measure the aortic valve annulus.
Evaluation of the aortic root dimensions and spatial relationship with surrounding structures
The evaluation of the aortic root dimensions along with the spatial relationships with surrounding structures such as coronary arteries are also crucial steps in the evaluation of the patients who are candidates for TAVI.
The measurement of the aortic root dimensions, including the diameters of sinus of Valsalva, sino-tubular junction and ascending aorta, is crucial to comprehensively evaluate patients who are candidates for TAVI.22 For example, the presence of dilated sino-tubular junction (>43 mm) contraindicates the implantation of the self-expandable prosthesis CoreValve ReValving System.9 Echocardiography is the most frequent used imaging technique to evaluate the dimensions of the aortic root. However, 3-dimensional imaging techniques such as MDCT and MRI are considered the gold standard methods. With MDCT, the use of multiplanar reformation planes permit definition of the true cross-sectional plane of the aortic valve annulus, sinus of Valsalva, sino-tubular junction and ascending aorta, providing high accurate measurements of the diameters of the aortic root components and ascending aorta (Figure 3).25,30 In addition, Burman et al recently provided the reference values for the aortic root measurement with the use of MRI.29 Whether these measurements may change the decision making of patients who are candidates for TAVI will need additional studies.
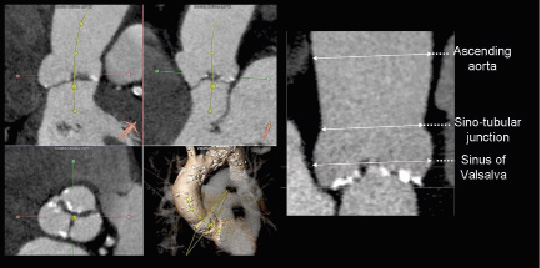
Figure 3. Measurement of the aortic root dimensions. The accurate alignment of the orthogonal planes with MDCT allows for accurate measurement of the diameters of the sinus of Valsalva, sino-tubular junction and ascending aorta (3mensio Valves™, version 4.1; 3mensio Medical Imaging BV, Bilthoven, The Netherlands). The panels on the left show the orientation of the centreline and the transversal plane providing accurate sizing of the aortic root in the stretched view (Panel on the right).
In addition, the measurement of the height of the coronary ostia relative to the aortic valve annular plane constitutes an important requirement before TAVI. In this regard, MDCT is a comprehensive imaging technique that also permits the evaluation of this parameter (Figure 4).22 The minimum distance between the coronary ostia and the aortic valve annular plane should be ≥13-14 mm, if the CoreValve ReValving System is used.31 Nevertheless, this transcatheter valve prosthesis can be implanted in patients with coronary ostia located at least 10 mm above the valvular annular plane if the native valve is not heavily calcified and the sinus of Valsalva is wide enough (≥27 mm). For Edwards SAPIEN the coronary ostia should be located ≥10-11 mm above the valvular annular plane.31 Normal in vivo distribution of the coronary ostia locations has been evaluated in several studies. The mean height of the left coronary ostium is around 15 mm whereas the right coronary ostium is located at a mean distance of 17 mm.12,32 Based on these results, the risk of coronary ostium occlusion is low. In addition, the design of the transcatheter prosthetic valves ensures the patency of the coronary flow through the free struts of the devices.
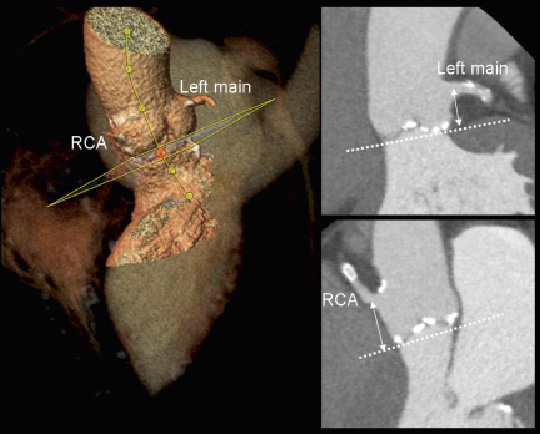
Figure 4. Height of the coronary ostia relative to the aortic valve annular plane. MDCT permits correct orientation of the aortic valve annular plane and, subsequently, accurately measure the distance between the aortic valve annular plane and the coronary ostia (3mensio Valves™, version 4.1; 3mensio Medical Imaging BV, Bilthoven, The Netherlands). RCA: right coronary artery
Finally, the assessment of the spatial orientation of the aortic root and the aortic valvular plane before the procedure is of interest. Recent studies have demonstrated that MDCT permits identification of the most appropriate fluoroscopic projections for the aortic root angiogram during TAVI (Figure 5).30 In a series of 40 patients with severe aortic stenosis, the conventional X-ray angiographic projections of the aortic root were compared to the 3-dimensional MDCT reformation planes. In the left anterior oblique projection, there were no differences in the angulations of the aortic root on conventional angiogram or MDCT (cranial: 25±7º vs. 23±8º, p=0.2). In contrast, for the right anterior oblique projection, there were significant differences between the two approaches (caudal: 21±9º for conventional angiography vs. 27±10º for MDCT, p=0.002).30 This analysis may have important clinical implications, reducing significantly the procedural timings and the volume of contrast used during TAVI.
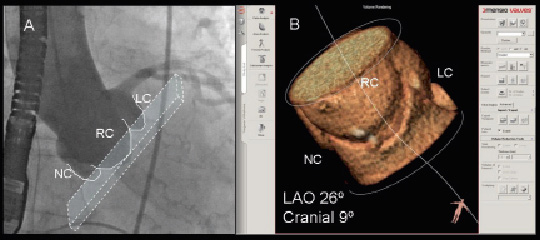
Figure 5. Multidetector row computed tomography to evaluate the aortic root orientation in the conventional fluoroscopic projections. The most appropriate fluoroscopic projections to align the aortic valve annular plane (Panel A) can be anticipated with the use of MDCT (Panel B). In the example, the most appropriate angulations on conventional fluoroscopic projections would be left anterior oblique 26º and cranial 9º. Post-processing imaging software: 3mensio Valves™, version 4.1; 3mensio Medical Imaging BV, Bilthoven, The Netherlands.
Peripheral vascular access evaluation: key step to select the TAVI approach
Currently, TAVI is performed through two different approaches: transarterial (transfemoral/ transaxillary/subclavian) and transapical approach. The transfemoral approach is the preferred technique due its less invasive character. However, the prevalence of concomitant peripheral vascular disease in patients with severe aortic stenosis is relatively high, increasing the risk of major vascular complications if the transfemoral approach is used.15 Therefore, in those cases, the transapical approach constitutes a feasible alternative.
In order to plan the most suitable procedural approach, accurate evaluation of peripheral arteries and thoracic aorta is crucial in the preprocedural evaluation. The CoreValve ReValving System requires a minimum luminal diameter of the ilio-femoral arteries of 6 mm. Recent developments in delivery systems have allowed the implantation of Edwards SAPIEN valve using a 18 Fr sheath (NovaFlex, Edwards Lifesciences) and, consequently, the required minimum diameter of the femoral arteries is also 6 mm. Invasive angiography can be used as a first screening imaging modality to measure the luminal diameter of the ilio-femoral arterial system.33 However, one of the limitations of this technique is the poor resolution of the soft tissue structures that limits its accuracy to evaluate the arterial wall. Particularly, the presence of circumferential and bulky calcification of the arterial wall may increase the risk of vascular complications during the procedure and may contraindicate the transfemoral approach. In contrast, MDCT provides meaningful information on the luminal dimensions, tortuosity of the peripheral arteries and the extent and location of vascular calcifications.14,15 With multiplanar reformation planes, the cross-sectional area of the arterial lumen can be accurately measured (Figure 6A). In addition, the oblique transversal view and the stretched views of the arteries permit the evaluation of the distribution of the calcifications along the vessel (Figure 6B). Finally, the 3-dimensional volume-rendered images permit evaluation of the tortuosity of the vessels (Figure 6C). Joshi et al recently demonstrated the accuracy of intra-arterial contrast-injection MDCT to evaluate the anatomy of the aorto-ilio-femoral arteries in 37 patients.14 Unfavourable peripheral artery anatomy was considered when the cross-sectional diameter of the arterial lumen was <7 mm, the presence of severe tortuosity (>135º change in vessel course) or circumferential arterial wall calcification. A good correlation between MDCT and invasive angiography measurements was observed (r=0.92, p<0.001) although MDCT systematically underestimated the luminal diameter by 1.0±1.6 mm (p<0.001).14 Based on MDCT analysis, the interventional approach would be changed in 43% of the patients. Nevertheless, the lower iodinated-contrast load used in intra-arterial contrast-infection MDCT compared to conventional angiography or conventional MDCT makes this approach suitable for patients with renal failure.
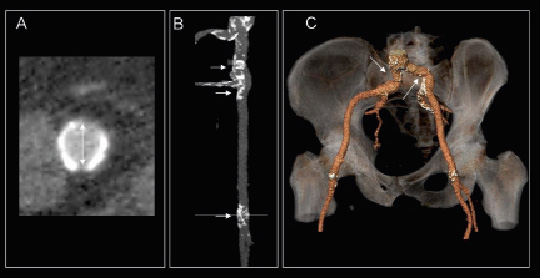
Figure 6. Evaluation of peripheral arteries with multidetector row computed tomography. The double oblique transversal view (Panel A) allows for the assessment of the internal luminal diameter and area and shows the distribution of the calcium along the curvature of the vessel. In addition, the stretched view (Panel B) shows the extent and location of the calcifications along the artery whereas the 3-dimensional volume render (Panel C) provides useful information on the presence of tortuous arterial segments that may challenge the transfemoral approach. Post-processing imaging software: 3mensio Valves™, version 4.1., 3mensio Medical Imaging BV, Bilthoven, The Netherlands.
In addition, the luminal diameter of the peripheral arteries and the presence of mural thrombosis can be evaluated with contrast-enhanced magnetic resonance angiography.13 The addition of true-FISP sequence acquisition to contrast-enhanced gadolinium magnetic resonance angiography results in improved image quality of the arterial wall showing the presence of mural thrombosis.13 Importantly, the acquisition of true-FISP sequences without use of paramagnetic contrast enables also the assessment of the luminal size, being a suitable alternative in patients with renal failure.
Finally, transesophageal echocardiography permits also the evaluation of the aortic wall and the detection of significant atherosclerosis. Significant atherosclerosis of the thoracic aorta may contraindicate the transfemoral approach due to the high risk of embolism during intra-aortic manipulation of the catheters. In addition, intravascular ultrasound imaging may be a valuable technique to accurately measure the luminal diameter of the peripheral arteries and to detect extensive atherosclerotic lesions of the arterial wall.
In summary, in patients with favourable anatomy of the peripheral arteries and aorta, the transfemoral access is the approach of choice to implant the transcatheter aortic valve. However, the presence of any unfavourable anatomical characteristic in patients with borderline arterial dimensions indicates the use of a transapical or trans-subclavian approach rather than transfemoral approach.
Other factors to be evaluated before TAVI
Additionally to the assessment of peripheral arteries and measurement of the aortic valve annulus and aortic root dimensions, other several aspects should be evaluated in the pre-procedural screening:
Coronary artery anatomy. Evaluation of coronary artery anatomy is mandatory before TAVI. The presence of significant coronary artery disease amenable to percutaneous coronary intervention should be evaluated before TAVI. Conventional invasive angiography is the reference imaging technique to evaluate coronary anatomy. In addition, MDCT may provide meaningful information in this regard. Several studies have demonstrated the accuracy of MDCT to detect coronary artery disease.34,35 However, in elderly populations the presence of highly calcified coronary arteries may limit the accuracy of this technique to identify significant arterial stenosis.
Left ventricular dimensions and function. Echocardiography remains as the cornerstone technique to evaluate left ventricular dimensions and function. Left ventricular hypertrophy is common in patients with severe aortic stenosis. Particularly, the presence of pronounced sigmoid basal septum may challenge the TAVI procedure. In these cases, the transapical approach may be preferred over the transfemoral approach since the former provides more stable positioning of the prosthesis.9 In addition, left ventricular ejection fraction is one of the strongest prognostic determinants in patients with severe symptomatic aortic stenosis.36 Accurate assessment of left ventricular function is mandatory to evaluate the operative risk of patients with symptomatic severe aortic stenosis. In patients with poor acoustic windows, the use of contrast media for left ventricular cavity opacification improves endocardial border identification and increases the accuracy of this technique in the assessment of left ventricular dimensions and function. In addition, contrast-enhanced echocardiography permits the identification of intracavitary thrombus, an established contraindication for TAVI. Finally, MDCT enables accurate assessment of left ventricular dimensions and function and permits the detection of intracavitary thrombus. Furthermore, the use of novel post-processing imaging software allows us to anticipate and plan the transapical approach (Figure 7). These advances may reduce the procedural and the fluoroscopy timings. Additional prospective studies will be warranted to evaluate the clinical implications of the use of these novel technologies.
Mitral valve regurgitation. Presence of concomitant significant mitral valve regurgitation may be also evaluated before TAVI. Particularly, a low implantation of the CoreValve ReValving system may interfere with the motion of the mitral valve (anterior mitral leaflet) and increase the severity of the regurgitant lesion.31 Nevertheless, recent experiences with this prosthetic valve have demonstrated that in the majority of patients the severity of mitral valve regurgitation did not significantly changed after TAVI.37
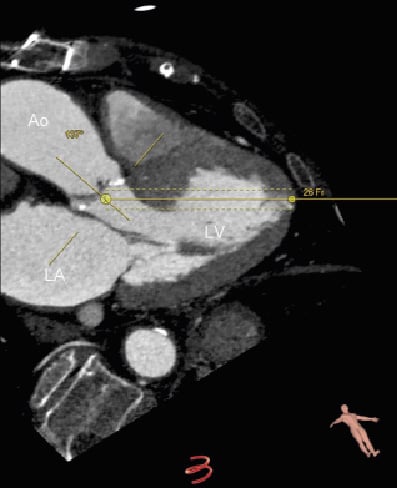
Figure 7. Assessment of left ventricular geometry before transapical TAVI. Novel advances in MDCT post-processing imaging permit visualisation of the left ventricular geometry and positioning relative to the aorta (3mensio Valves™, version 4.1., 3mensio Medical Imaging BV, Bilthoven, The Netherlands). The angle between the aortic root and the left ventricle may be of relevance before planning TAVI through transapical approach. The simulator allows us to anticipate the location of the delivery system into the left ventricle and how to position the prosthesis into the aortic valve. Ao: aorta; LA: left atrium; LV: left ventricle
Conclusions and summary check-list
The encouraging results of the first series of patients treated with TAVI have confirmed this therapeutic technique as a feasible alternative to conventional surgery in those patients with high operative risk. However, the success and procedure-related complications rates depend on accurate preprocedural assessment of the patients who are candidates for TAVI. Multimodality imaging plays a central role in this preprocedural evaluation. In summary, the key steps that should be accurately evaluated include:
1. Confirmation of the aortic stenosis severity
2. Assessment of aortic valve anatomy and extent of calcifications
3. Measurement of aortic valve annular size
4. Measurement of aortic root dimensions and left ventricular outflow tract
a. Sinus of Valsalva
b. Sino-tubular junction
c. Ascending aorta
5. Height of the coronary ostia relative to the aortic valve annular plane
6. Evaluation of vascular access: peripheral arteries and thoracic aorta
a. Luminal diameter
b. Tortuosity
c. Calcifications (porcelain aorta)
7. Coronary artery anatomy
8. Left ventricular dimensions and function and presence of intracavitary thrombus
9. Mitral regurgitation.
MDCT may constitute the most comprehensive imaging technique to evaluate all these aspects. However, radiation exposure and the use of iodinated-based contrast agents may limit its use in young populations and patients with renal insufficiency, respectively. In those cases, MRI may be a feasible alternative to evaluate patients who are candidates for TAVI. Table 1 summarises the aforementioned parameters that should be taken into account during the preprocedural evaluation and indicates the most appropriate imaging technique to evaluate them. In addition, the anatomical requirements for the transcatheter aortic prostheses are included. In addition, Figure 8 illustrates a proposal for check-list measurements based on MDCT as this imaging technique may provide the most comprehensive assessment of patients who are candidates for TAVI.
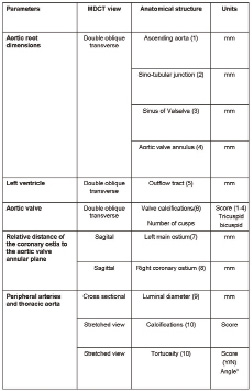
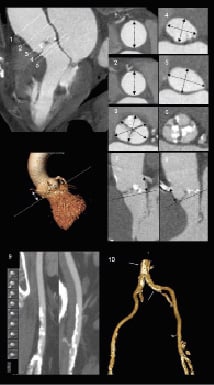
Figure 8. Check-list of measurements before TAVI based on MDCT. The table summarises the key measurements before TAVI including the aortic root dimensions, the aortic valve annular diameters, left ventricular outflow tract dimensions, extent of valvular calcifications, distance of the coronary ostia relative to the aortic valve annular plane and evaluation of the peripheral arteries (diameters, calcifications and tortuosity). Some of these measurements can be performed with other imaging techniques (echocardiography or MRI). More important, standardisation of the preprocedural evaluation following the proposed check-list may help to accurately select candidates for TAVI.

