Up until 2010, paravalvular aortic regurgitation (pAR) of any severity was diagnosed in 70-90% of patients after transcatheter aortic valve implantation (TAVI) using either a self- or balloon-expanding prosthesis; moderate to severe pAR was observed in 10-30%1. During the last two years, moderate to severe pAR has been documented in 0-12% of patients undergoing TAVI (Figure 1), including those patients implanted with the Medtronic CoreValve (Medtronic, Minneapolis, MN, USA), a valve which has not undergone significant iterations until recently. What can explain the significant reductions in pAR over time?
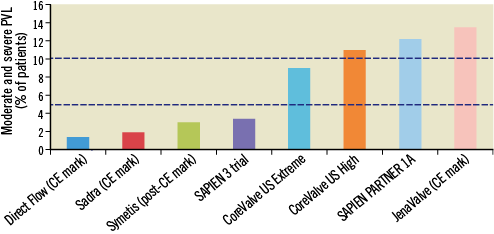
Figure 1. Rates of significant paravalvular aortic regurgitation (>2+) across various transcatheter aortic valve platforms in contemporary CE mark or post-market registry and FDA trials. The two horizontal dotted lines represent 5 and 10% rates of paravalvular aortic regurgitation (for reference).
From the first TAVI in 2002 to 2010, two-dimensional (2D) echocardiography was the gold standard imaging modality for transcatheter aortic valve sizing. In the last two years, we have witnessed a rapid and dramatic shift to multislice computed tomography (MSCT)-based transcatheter aortic valve sizing. Amongst other less influential factors, this shift to MSCT appears to explain the significant reductions in pAR over time. Although randomised comparisons are lacking, observational studies strongly suggest the superiority of MSCT over 2D echocardiography for correct valve sizing. There is an ongoing debate about the relative benefits of MSCT-measured annular “perimeter” versus “area” for valve sizing.
With this in mind, there are a number of significant hurdles in the acquisition, interpretation and application of MSCT data: (1) imaging hardware and software; (2) MSCT acquisition protocol; (3) multiplanar reconstruction methodology, and (4) correct application of measurements to the various transcatheter aortic valve platforms. Although these may seem like trivial factors, they significantly limit the utility and benefit of MSCT in everyday clinical practice, outside the clinical trial environment.
Stortecky et al have evaluated a dedicated MSCT software-imaging tool in terms of its accuracy and reproducibility in measuring the aortic annulus2. Furthermore, they examined the interdependence, amongst various measurements, of the aortic annulus obtained by MSCT, 2D echocardiography and angiography.
Although the results of this study were not necessarily surprising, they were reassuring. The authors found excellent correlation between two independent and blinded observers for all annulus measurements using the dedicated MSCT reconstruction software. In order to achieve excellent correlation amongst users, it is imperative that the MSCT reconstruction methodology is of course reproducible. Two important factors are at play here: (1) the authors have correctly described and implemented the steps to identify and measure the annulus, and (2) the dedicated software is partially “automatic” and helps eliminate potential user differences. This particular dedicated software is being used extensively in everyday clinical practice and trials, and this study by Storecky et al provides a reliable reference for its use.
The comparison of annulus diameter measurements obtained by various imaging techniques (echocardiography, angiography, and MSCT) confirms the results from previously published reports; namely, annular measurements obtained from 2D echocardiography are on average smaller than from angiography and those obtained by MSCT-sagittal are smaller than by MSCT-coronal. Before the vast amount of insights into the enigmatic aortic annulus, it was not uncommon to select a transcatheter aortic valve size based on 2D echocardiography and subsequently change our minds for a larger valve size during the procedure based on contrast aortography. The explanation behind this lies in the fact that, from an attitudinal position, the major and minor axis of the aortic annulus can be viewed from anteroposterior (coronal) and right-left (sagittal) views, respectively. In essence, the parasternal long-axis echocardiographic view corresponds to the MSCT sagittal view and the “anterior-posterior” contrast aortography view corresponds to the MSCT coronal view.
The authors suggest that the annulus is “primarily oval in shape”. Perhaps the annulus is best described as polygonal. This would rightfully prevent the assumption of a “major” and “minor” axis as it relates to an ellipse. Furthermore, it would avoid the erroneous calculation of an “area-derived” or “perimeter-derived” diameter using circular equations that can have negative effects on selecting the appropriate valve size. The measured perimeter or area should be directly related to the nominal valve perimeter or area to best understand the potential percent oversizing or interference (Figure 2). In the context of transcatheter aortic valve sizing, we should refrain from further discussions involving a particular diameter, whether measured or derived.
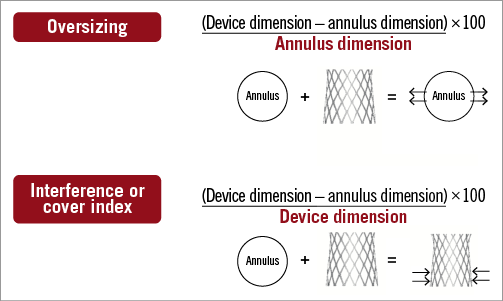
Figure 2. The concept of oversizing versus interference is described. Transcatheter aortic valves may be remodelled by the annulus (i.e., their expansion is being “interfered” with) while other transcatheter aortic valves remodel the annulus (i.e., the prosthesis is “oversized” with respect to the annulus). This has implications when calculating percent interference or oversizing.
The authors correctly noted the absence of three-dimensional (3D) echocardiography as a limitation of the current study. Similar to MSCT, 3D echocardiography allows appreciation of the annular perimeter or area. More recently, Khalique et al have shown comparable discriminatory power between MSCT and 3D echocardiography for the identification of significant pAR3. It is potentially foreseeable that we move “back to the future” and make 3D echocardiography the gold-standard imaging modality of choice for transcatheter aortic valve sizing. The advantages of echocardiography include its lack of radiation exposure, lack of contrast use, lower costs and logistic implications. Nonetheless, in the current state of art, the spatial resolution of 3D echocardiography is inferior to MSCT, and future studies are needed to better understand the relative benefits and limitations of MSCT and 3D echocardiography.
Despite the importance we give to the measurement of the aortic annulus in transcatheter aortic valve sizing, it is not just about the annulus. The left ventricular outflow tract, sinus of Valsalva, sinotubular junction and the extent and distribution of calcium across the aortic valvar complex need to be appreciated as well!
Conflict of interest statement
N. Piazza has received research grants from Medtronic and St. Jude, is a proctor for Medtronic and a consultant for Medtronic HighLife and CardiaQ. P. Thériault-Lauzier has no conflicts of interest to declare.

