Abstract
Paravalvular aortic regurgitation (AR) has a negative impact on the prognosis following transcatheter aortic valve implantation (TAVI). As transcatheter heart valves (THV) are implanted in a sutureless fashion using oversizing to anchor the prosthesis stent frame at the level of the virtual aortic annulus, incomplete stent frame expansion due to heavily calcified cusps, suboptimal placement of the prosthesis, and/or annulus-prosthesis size mismatch due to malsizing can contribute to paravalvular AR with increased mortality in patients with more than mild paravalvular AR. Echocardiography is essential to differentiate between transvalvular and paravalvular AR and to elucidate further the aetiology of AR during the procedure. However, since echocardiographic quantification of AR in TAVI patients remains challenging especially in the implantation situation, a multimodal approach for the evaluation of AR with use of haemodynamic measurements and imaging modalities is imperative to quantify the severity of AR precisely immediately after valve implantation. Thus, patients who will benefit from corrective measures such as post-dilation or valve-in-valve implantation can be identified. In these patients, every measure has to be taken to reduce paravalvular aortic regurgitation in order to improve outcome.
Abbreviations
AR: aortic regurgitation
AR index: aortic regurgitation index
DBP: diastolic blood pressure
LVEDP: left ventricular end-diastolic pressure
PVL: paravalvular leakage
SAVR: surgical aortic valve replacement
SBP: systolic blood pressure
TAVI: transcatheter aortic valve implantation
THV: transcatheter heart valve
Introduction
Transcatheter aortic valve implantation (TAVI) has been shown to be non-inferior compared to surgical aortic valve replacement (SAVR) for surgical high-risk patients with severe aortic stenosis, and to be superior to conservative management in inoperable patients in the randomised PARTNER trial1-3. Transcatheter heart valves (THV) are implanted in a sutureless fashion using oversizing to anchor the prosthesis at the level of the native aortic annulus. Therefore, incomplete circumferential apposition of the prosthesis with the annulus, which can be caused by incomplete stent frame expansion due to heavily calcified cusps, suboptimal placement of the prosthesis, and/or annulus-prosthesis size mismatch due to malsizing, might lead to paravalvular aortic regurgitation (AR). Since paravalvular AR has a negative impact on the prognosis following TAVI with increased morbidity and mortality in patients suffering from more-than-mild paravalvular AR, this procedure-related shortcoming has to be addressed to provide satisfying long-term clinical outcome4-12.
This paper focuses on the precise quantification of significant paravalvular AR in TAVI patients and therapeutic options to manage paravalvular AR following TAVI.
Paravalvular aortic regurgitation
Several studies have demonstrated that up to 70% of all TAVI patients suffer from paravalvular AR after the procedure - in approximately 15 to 20% of the patients graded more than mild11,12. It has been shown that the occurrence of more-than-mild paravalvular AR negatively impacts on the prognosis following TAVI with an up to fourfold increased one-year mortality risk compared to patients without clinically significant paravalvular AR4-10,13-17. In line with the secondary analysis of the two-year data from the PARTNER 1A trial9, a recent review even suggested that a lesser extent of paravalvular AR may be harmful for TAVI patients, so that mild paravalvular AR might have a negative impact on prognosis11. Therefore, differentiation between transvalvular and paravalvular AR, precise quantification of the severity of AR in the acute implantation situation, and identification of the underlying mechanism with imaging modalities are essential to identify patients for whom corrective measures will be beneficial (Figure 1).
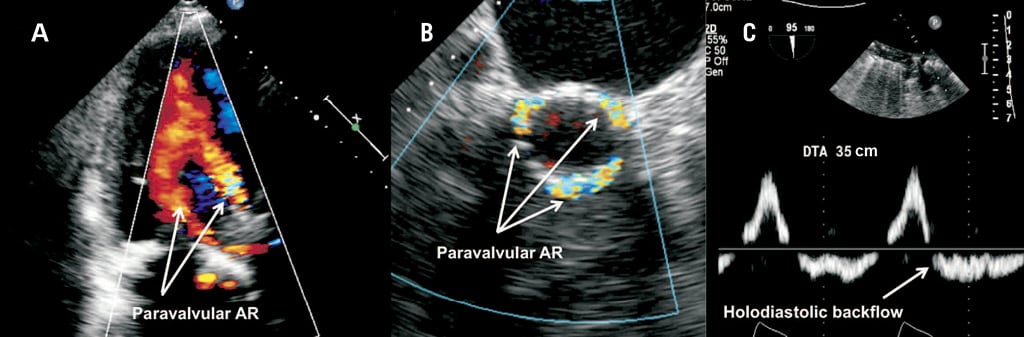
Figure 1. Echocardiographic assessment of paravalvular aortic regurgitation. Colour transthoracic echocardiography (TTE) 3-chamber view of a CoreValve 31 mm prosthesis with too low ventricular placements leading to severe paravalvular aortic regurgitation (AR) (A). Colour 3-D TEE (transoesophageal echocardiography) of malposition-related PAR following low implantation of a Medtronic CoreValve prosthesis. The PAR jet passes from within the aortic portion of the stent frame above the tissue skirt (“supra-skirt” PAR) into the paravalvular space and LVOT (B). Prominent holodiastolic backflow in the descending aorta indicating clinically significant paravalvular AR (C).
Angiographic assessment of paravalvular AR (PAR)
The angiographic grading of PAR is only qualitative, since the regurgitant flow within each angiographic grade varies widely, and a considerable overlap from one grade to another has been found18,19. PAR can be classified according to the visually estimated density of opacification of the left ventricle (LV) into three degrees adapted to the VARC-2 criteria20: mild (reflow of contrast in the outflow tract and middle portion of the LV but clearing with each beat), moderate (reflow of contrast in the whole left ventricular cavity with incomplete washout in a single beat and faint opacification of the entire LV over several cardiac cycles), and severe (opacification of the entire LV with the same intensity as in the aorta and persistence of the contrast after a single beat). Additionally, in patients with chronic renal failure and/or at high risk for the development of acute kidney injury, the required use of contrast dye is disadvantageous.
Echocardiographic assessment of paravalvular AR
The echocardiographic quantification of paravalvular AR - especially in the acute implantation situation - remains challenging despite the recently updated VARC-2 criteria20. Most parameters refer to recommendations for surgical prosthetic heart valves which have not yet been validated for THVs21. For the following reasons, the grading of paravalvular AR remains imprecise: 1) acute haemodynamic changes including heart rate during the procedure confound Doppler and colour flow assessment; 2) semi-quantitative parameters of AR severity such as jet width, vena contracta or pressure half time (PHT) are not ideal for the quantification of eccentric, circumferential paravalvular AR jets, which are observed in TAVI patients; and 3) acoustic shadowing by the prosthesis and calcifications of the native aortic valve may also obscure paravalvular AR jets. Given these quantitative limitations, transoesophageal echocardiography (TEE) screening criteria include a jet depth extending beyond the LVOT, the circumferential extent of the AR jet in a short-axis view (<10%: mild, 10-29%: moderate, and ≥30%: severe PAR), and holodiastolic flow reversal in the descending aorta12,20,21. Nonetheless, TEE has an essential role in differentiating between transvalvular and paravalvular AR and defining the underlying aetiology of paravalvular AR. Paravalvular AR jets, which are caused by malapposition between the prosthesis and the annulus due to heavy calcification or underexpansion of the prosthesis, occur outside the circumference of the prosthetic stent frame. Furthermore, “supraskirt” and “infraskirt” AR jets, which are caused by too deep or too shallow positioning of the prosthesis, respectively, have to be differentiated to guide further treatment12.
Haemodynamic assessment of paravalvular AR
Haemodynamic parameters for a quantitative evaluation of PAR have been shown to be useful in two recent studies4,8. The so-called “aortic regurgitation index” is the ratio of the end-diastolic, transvalvular gradient between diastolic blood pressure (RRdia) in the aorta and left ventricular end-diastolic pressure (LVEDP) to systolic blood pressure (RRsys) in the aorta: [(RRdia – LVEDP) / RRsys] x 100. In both cohorts, the AR index showed an inverse association with the degree of AR and helped to differentiate among patients suffering from mild, moderate, or severe paravalvular AR. In addition to aortography or echocardiography, the AR index is a helpful tool to identify patients after TAVI, for whom corrective measures should be taken to decrease the severity of paravalvular AR. However, the AR index still has to be validated in a larger and controlled study population, as being applicable for angiography and echocardiography as well.
To overcome the limitations of the measurement of haemodynamics, it is recommended to determine the dimensionless AR index approximately 10 minutes after valve deployment to prevent confounding by an increased LVEDP due to myocardial ischaemia after rapid pacing and balloon valvuloplasty. Furthermore, the AR index, as with AR and imaging modalities in general, is dependent on the heart rate and thus should be determined as mean value over several cardiac cycles (especially in patients suffering from atrial fibrillation) with a heart rate of 60-80 bpm and without extrasystolic beats. With increasing heart rate and shortened duration of the diastole, the diastolic pressure in the aorta also increases and thereby might incorrectly lead to an AR index above the cut-off of 2512.
Treatment options to reduce PAR
Data on corrective measures, which have been proposed to reduce significant residual paravalvular AR after TAVI, predominantly originate from smaller series, and the impact of these corrective measures on long-term outcome and valve durability still has to be clarified in future studies22-25. Recently, we recommended a multimodal algorithm for the management of paravalvular AR after TAVI12. After valve deployment, the degree of paravalvular AR should be assessed by aortography and/or echocardiography. If mild to severe paravalvular AR occurs after TAVI, the determination of the AR index helps to quantify more precisely the extent of paravalvular AR, and provides a quantitative reference before corrective measures are taken. In patients with more-than-mild PAR and/or an AR index <25, the evaluation of paravalvular AR by echocardiography, preferably TEE, is recommended to elucidate the aetiology of paravalvular AR. Thus, patients with the need to take corrective measures such as post-dilation or valve-in-valve implantation to reduce paravalvular AR can be identified (Figure 2). When corrective measures have been taken in patients with clinically significant PAR, the severity of PAR can be re-evaluated by imaging modalities and the AR index.
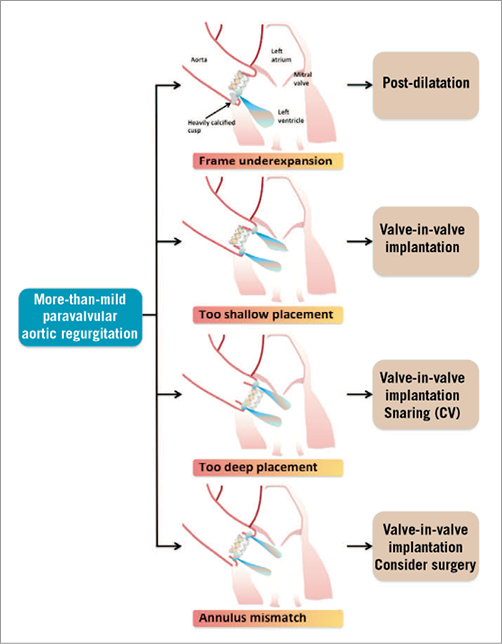
Figure 2. Treatment options according to the aetiology of paravalvular AR after TAVI. For the treatment of more-than-mild paravalvular AR, several treatment options exist according to the aetiology of AR: 1) post-dilation for frame underexpansion; 2) valve-in-valve implantation for too shallow placement of the prosthesis; 3) for too deep placement of the prosthesis, valve-in-valve implantation or a snaring manoeuvre in selected patients after implantation of a Medtronic CoreValve; and 4) valve-in-valve implantation or (bail-out) cardiac surgery has to be considered for patients with annulus-prosthesis mismatch.
Balloon post-dilation
Heavy calcification of the native aortic valve or the LVOT might lead to suboptimal frame expansion leading to a typical eccentric AR jet (Figure 3). Several studies identified that the severity of native aortic valve calcification is related to the occurrence of more-than-mild paravalvular AR26,27. Balloon post-dilation reduces the degree of paravalvular AR by obtaining a better expansion of the prosthesis stent frame and a better sealing of the paravalvular space if the THV has been deployed at correct implantation depth22,24,25. Post-dilation is also the treatment option of choice for patients with inversion of the prosthesis stent frame (as the reason for severe PAR) that can occur in rare cases – despite predilation of the native aortic valve – with the use of self-expanding THVs28.
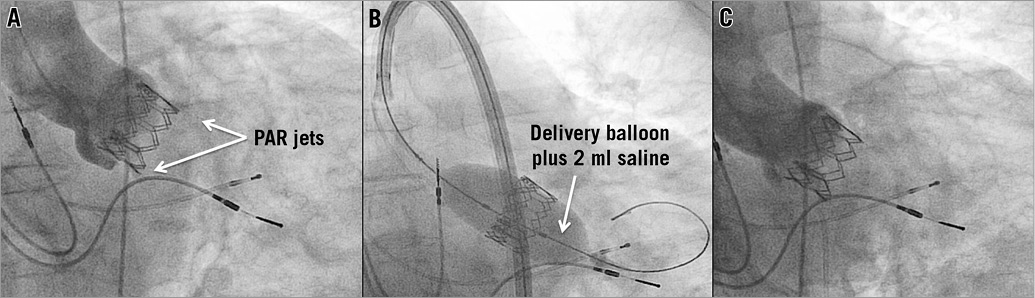
Figure 3. Balloon post-dilation. Underexpansion of an Edwards SAPIEN 26 mm prosthesis due to a severely calcified cusp resulted in moderate paravalvular aortic regurgitation (PAR) with an eccentric jet near the left coronary cusp in aortic root angiography (A). Post-dilation with the delivery balloon plus two millilitres of saline added to the total volume to increase its diameter (B) led to a satisfying procedural result with only mild PAR (C).
The size of the balloon for post-dilation should conform to the aortic annulus dimension. For the Medtronic CoreValve prosthesis (Medtronic, Minneapolis, MN, USA), a straight valvuloplasty balloon with a maximum diameter of 22, 25, 28, and 30 mm is recommended for the 23, 26, 29, and 31 mm CoreValve, respectively12. For the Edwards SAPIEN prosthesis (Edwards Lifesciences, Irvine, CA, USA), balloon post-dilation should be performed stepwise with the same balloon as used for delivery of the valve prosthesis, adding one millilitre of saline to the total volume to increase its diameter25.
Valve-in-valve implantation
Suboptimal deployment with malpositioning of the THV can result in incomplete sealing of the native aortic annulus by the pericardial skirt of the stent frame, allowing diastolic backflow into the left ventricle. In patients with malpositioned THVs with too shallow or too deep implantation of the prosthesis, valve-in-valve implantation can be a treatment option to reduce significant PAR and to prevent bail-out cardiac surgery (Figure 4). The second valve is deployed so that the sealing pericardial skirts of both valves overlap and to ensure sealing with the native aortic annulus23,29,30. Thus, initial procedural failure can be converted into procedural success in up to 90% of cases23,29,30. Furthermore, the valve-in-valve technique is a viable treatment strategy for significant transvalvular AR due to severe prosthetic leaflet dysfunction and for late failure of THVs, also in case of restenosis31,32.
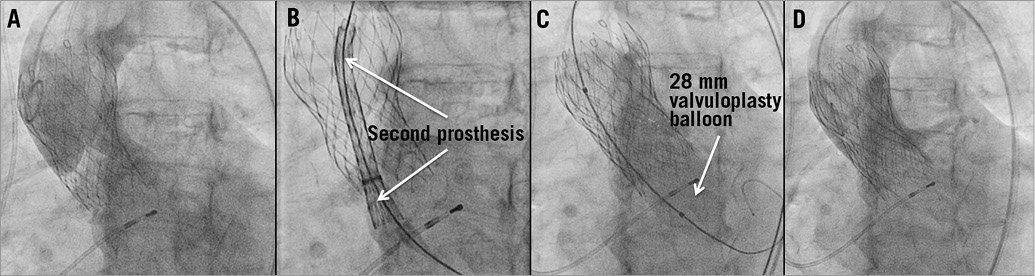
Figure 4. Valve-in-valve implantation. Very high implantation of a CoreValve 29 mm prosthesis led to embolisation of the prosthesis during the release from the delivery catheter with subsequent severe paravalvular aortic regurgitation in aortic root angiography (A). After implantation of a second CoreValve 29 mm prosthesis using the valve-in-valve-technique, which was delivered approximately 10 mm lower than the first CoreValve under fluoroscopic control (B,C), only mild PAR was left on angiography (D).
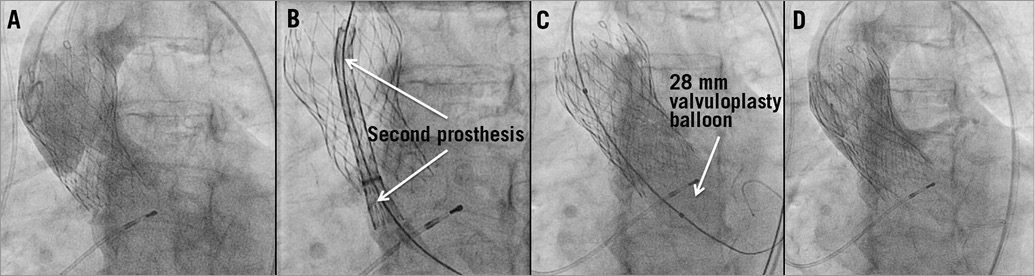
Figure 4. Valve-in-valve implantation. Very high implantation of a CoreValve 29 mm prosthesis led to embolisation of the prosthesis during the release from the delivery catheter with subsequent severe paravalvular aortic regurgitation in aortic root angiography (A). After implantation of a second CoreValve 29 mm prosthesis using the valve-in-valve-technique, which was delivered approximately 10 mm lower than the first CoreValve under fluoroscopic control (B,C), only mild PAR was left on angiography (D).
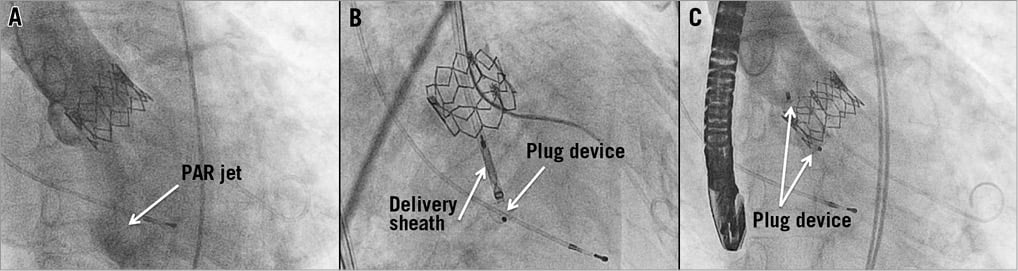
Figure 5. Interventional closure of paravalvular leakage. Despite balloon post-dilation and oversizing with a straight 25 mm valvuloplasty balloon, moderate AR due to incomplete circumferential apposition of an Edwards SAPIEN 26 mm prosthesis with the annulus (A) remained after implantation of this accurately sized prosthesis. Transoesophageal echocardiography (TEE) was used to identify the PAR pathomechanism and identified a localised paravalvular leak. After deployment of an AMPLATZER Vascular Plug III (B) under guidance with real-time three-dimensional TEE, angiography showed successful leak closure with only trace PAR left (C).
Snare technique
The snare technique represents a treatment strategy which may be considered in selected cases for a Medtronic CoreValve with too ventricular a placement of the prosthesis. Correction of the device position may be achieved by engaging one of the anchoring hooks and pulling with a snare catheter22. To increase the leverage effect, the snaring manoeuvre can be performed via transbrachial access. However, the snare technique lacks predictability and bears the potential risk of THV embolisation into the ascending aorta and can cause vascular complications (e.g., aortic dissection)4,12,24. If the snare technique fails, valve-in-valve implantation can be considered to prevent conversion to emergency open heart surgery.
Interventional closure
Interventional closure of paravalvular leaks after TAVI has been described for the Edwards SAPIEN prosthesis33,34 (Figure 5). If the implantation depth of the THV is appropriate and the THV is not undersized, balloon post-dilation can be the first step to obtain a better expansion of the prosthesis stent frame. If significant paravalvular AR remains due to heavy calcifications of the native aortic valve and a localised AR jet can be identified, transcatheter device closure with use of the AMPLATZER® Vascular Plug III (AVP III; AGA Medical Corp., Plymouth, MN, USA) can be attempted analogous to paravalvular leak closure in surgical heart valves35. However, potential risks associated with transcatheter device closure of paravalvular leaks following TAVI include stroke, THV dislodgement, and embolisation of the closure device34.
Summary
Paravalvular AR negatively impacts on outcome after TAVI. For the evaluation of paravalvular AR after TAVI, a multimodal approach with use of haemodynamic measurements and imaging modalities can be used to quantify more precisely the degree of AR immediately after valve implantation and to identify patients in whom corrective measures such as post-dilation or valve-in-valve implantation may be needed. Every measure has to be taken to prevent or reduce PAR in order to provide a satisfying long-term clinical outcome.
Conflict of interest statement
J.M. Sinning, N. Werner, and G. Nickenig receive speaker honoraria and research grants from Medtronic and Edwards Lifesciences. E. Grube is a proctor for Medtronic.

