
The clinical impact of paravalvular regurgitation (PVR) following surgical aortic valve replacement (SAVR) varies depending on the type of valve prosthesis, implant location, and means of delivery. In the aortic position, the incidence of prosthetic PVR is 2%-10%1. A meta-analysis of 12 clinical studies showed that a successful transcatheter closure of PVR (reduction of ≥1 grade) translated into lower cardiac mortality (odds ratio [OR] 0.08, confidence interval [CI]: 0.01-0.9) and greater improvement in New York Heart Association (NYHA) functional class or haemolysis (OR 9.95, CI: 2.1-66.7) with fewer repeat operations (OR 0.08, CI: 0.01-0.4)2. A random effects meta-analysis (including 604 patients from five observational studies) showed no significant difference between the two treatment strategies in terms of all-cause mortality (risk ratio 1.05, 95% CI: 0.63 to 1.76)3. Societal guidelines currently recommend transcatheter closure as an alternative to open surgery in patients with severe PVR at high risk for conventional surgery with anatomic features suitable for transcatheter therapy, after discussion with the Heart Team (class of recommendation IIa, level of evidence B)4,5.
Conventional cineangiography with an aortic root injection of radiographic dye has long been used for intraprocedural determination of the severity of aortic regurgitation (AR)6. However, standard angiographic grading, while helpful in extremes, may not correlate well with quantitative assessment of AR severity, and cannot reliably distinguish central from paravalvular regurgitation. In the Interventional Flashlight case report by Teixeirense et al7, the authors use quantitative videodensitometry (VD) to assess the severity of PVR before and after transcatheter closure of SAVR PVR. Although preprocedural assessment of SAVR PVR can be accomplished by computed tomography (CT), cardiac magnetic resonance (CMR) or echocardiography, intraprocedural guidance typically requires transoesophageal echocardiography (TEE)1, as was used by Teixeirense et al, obviating the need for additional imaging modalities for this entity. In addition, TEE is typically also required to determine the type and size of the PVR closure device1,8.
Thus, although there seems little need for quantitative VD for SAVR PVR, transcatheter aortic valve implantation (TAVI) is frequently performed under conscious sedation where the assessment of PVR by echocardiography falls to transthoracic imaging9, which may be limited by physical challenges (i.e., the supine position and access to optimal windows) as well as technical challenges (i.e., acoustic shadowing). Quantitative VD has been proposed as a more reproducible measure of AR following TAVI10,11,12. Unfortunately, the strict imaging protocol required (preprocedural determination of optimal projection angle [OPA], constant contrast infusion rate, optimal location of catheter tip, etc.) has previously limited the general applicability of this method. Using the aortic root as the reference region and using a limited region of the left ventricular outflow tract (LVOT) to reduce contrast overlap, a ratio of LVOT-to-aortic (LVOT:Ao) density ratio of >0.17 corresponded to >mild AR (defined by echocardiographic assessment); however, an average of four cycles improved accuracy11. Compared to regurgitant fraction by CMR, a VD LVOT:Ao ratio of ≥10% corresponded to >mild PVR, whereas a ratio of ≥25% corresponded to moderate-to-severe PVR12.
Quantitative VD, however, has a number of limitations in the high surgical risk and TAVI population. The assessment requires additional intraprocedural time to determine the OPA, a specific injection protocol that necessarily increases the contrast burden in each case, and fails to discriminate transvalvular regurgitation from PVR – important in determining the appropriate intraprocedural treatment following TAVI (i.e., post-dilatation or valve-in-valve). Intraprocedural haemodynamics have also been used13,14 but similarly suffer from the dependence on heart rate, an inability to distinguish central from paravalvular regurgitation and, in addition, significant overlap of mild and moderate PVR grades, the latter grade being associated with an increase in mortality post TAVI15.
Echocardiography thus remains the preferred method for assessing PVR, since this imaging modality can identify the location (including central versus paravalvular), number and size of the PVR jets, and provide a multi-parametric assessment in a continuous, physiologic manner. Although a 5-grade scheme has been proposed for research protocols16, this scheme can be reduced to the typical 3-grade scale advocated by the guidelines (Table 1). Whereas grading of surgical or transcatheter AR is similarly multi-window and multi-parametric, grading of PVR following TAVI has important caveats9:
1. Circumferential extent should be assessed as the sum of the individual small jets and not the arc that includes non-regurgitant spaces due to the stent frame or calcific leaflets. To assess the jet number and regurgitant orifice location and size accurately, meticulous scanning is required to identify the origin of the jets and confirm their path into the LVOT.
2. Given the frequent presence of ventricular and aortic compliance abnormalities in this patient population, mitral E:A ratio, pressure half-time and holodiastolic reversal of flow in the descending aorta should be used with caution in isolation but may have utility when comparing a baseline to a post-TAVI flow pattern.
3. Jet length or jet area should not be used to assess the severity of AR following TAVI since these jets are frequently eccentrically directed, constrained by the LVOT, or entrained within the LVOT, leading to rapid jet broadening.
4. Both quantitative Doppler and three-dimensional colour Doppler may be used to assess regurgitant orifice area and regurgitant volume, with the caveat that, in the acute setting, lower regurgitant volumes may be associated with haemodynamically severe PVR.
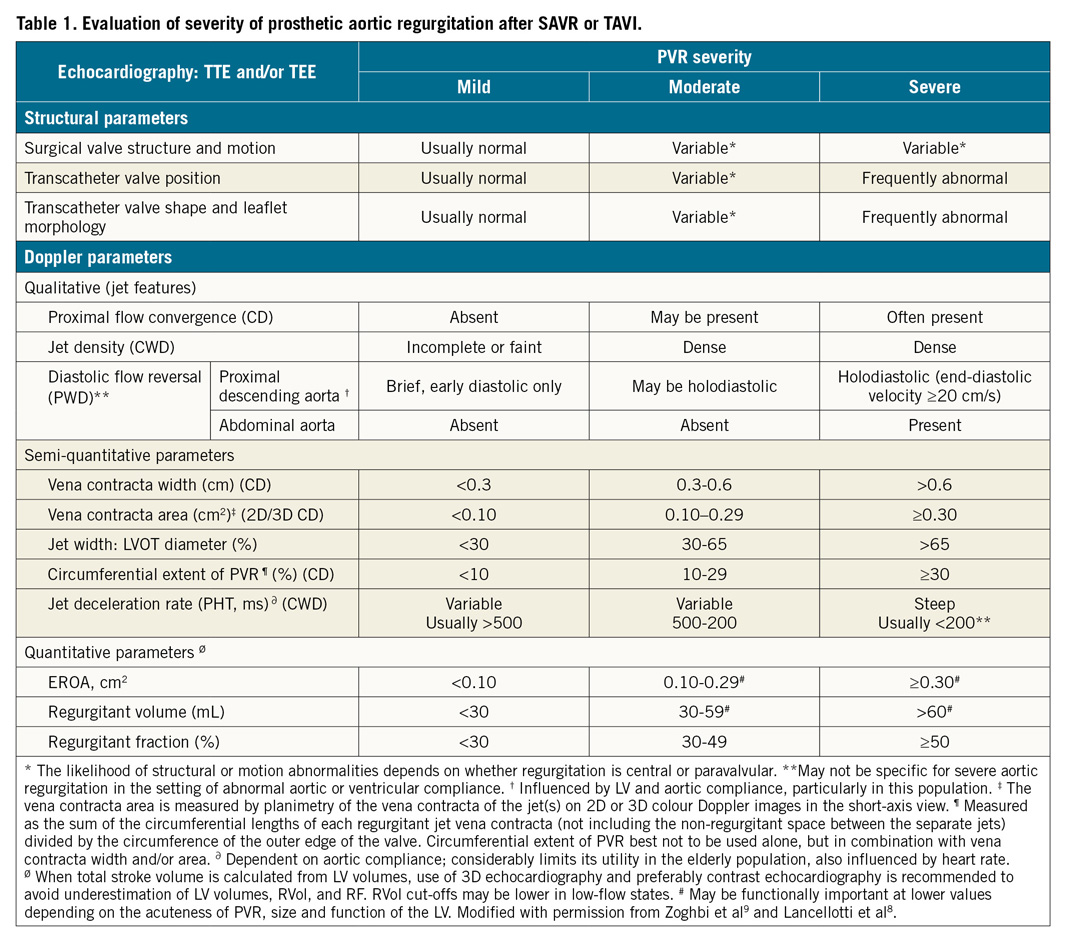
In summary, although quantitative VD is feasible, the overlap of AR grades, inability to localise the PVR jet or distinguish paravalvular from central regurgitation, and increased contrast use relegate it (along with haemodynamic measures) to an adjunctive tool, with echocardiography being the diagnostic test of choice in all guidelines. The echocardiographic quantitation of PVR is however nuanced, and a multi-window, multi-parametric assessment should be performed.
Conflict of interest statement
R. Hahn reports speaker fees from Boston Scientific Corporation and Baylis Medical; consulting for Abbott Structural, Edwards Lifesciences, Gore & Associates, Medtronic, NaviGate, Philips Healthcare and Siemens Healthcare; non-financial support from 3mensio and GE Healthcare. She is Chief Scientific Officer for the Echocardiography Core Laboratory at the Cardiovascular Research Foundation for multiple industry-sponsored trials, for which she receives no direct industry compensation.
Supplementary data
To read the full content of this article, please download the PDF.

