
More than a decade after the first successful transcatheter aortic valve implantation (TAVI), the field is rapidly moving towards perfecting the procedure to a level where it can be offered to all patients with aortic stenosis1,2. As the field matures, complications such as paravalvular aortic regurgitation, stroke, conduction abnormalities, vascular trauma, coronary compromise, and annular rupture have been targeted for improvement with better devices and sophisticated imaging.
In this issue of EuroIntervention, Stundl et al report their procedural strategy to address the most prevalent of these complications, paravalvular aortic regurgitation (AR) after TAVI3. Over the years, strategies for minimising paravalvular AR have evolved as our understanding of the underlying pathoanatomy has expanded4.
Recognition of the fact that even “mild” paravalvular AR is associated with increased long-term mortality was an important impetus to find solutions for eliminating AR5. Proper sizing of the devices by three-dimensional imaging was the most important step in reducing AR. The importance of accurate placement of the prosthesis in reducing AR was recognised early in the development of TAVI. Whilst improving the technique, innovations in devices for accurate positioning and effective sealing of the prosthesis annulus interface have also helped to minimise paravalvular AR6. The impact of all these efforts to reduce AR has been predominantly determined by quantifying AR using echocardiography. Over the years it has become increasingly clear that our methods of quantifying the severity of paravalvular AR have limitations. Proper quantification of AR is critical for the TAVI field to improve so that the indication for TAVI can be expanded to the treatment of low surgical risk patients. The manuscript by Stundl et al demonstrates the utility of haemodynamic measurement along with angiographic and echocardiographic assessment of AR at the time of TAVI.
The severity of paravalvular AR can be assessed by echocardiography (transthoracic [TTE] or transoesophageal [TEE]), angiography and haemodynamic measurements at the time of TAVI (Table 1). In subsequent evaluations, the severity of AR is typically reported using TTE, although in very small populations magnetic resonance imaging (MRI) has been used. Biomarkers like brain natriuretic peptide (BNP) and left ventricular mass or dimensions can be used as a surrogate for the severity of AR. All of these measurements have significant limitations and variability when used individually. However, when used together, each one adds a different facet to the understanding of the severity of paravalvular AR7.
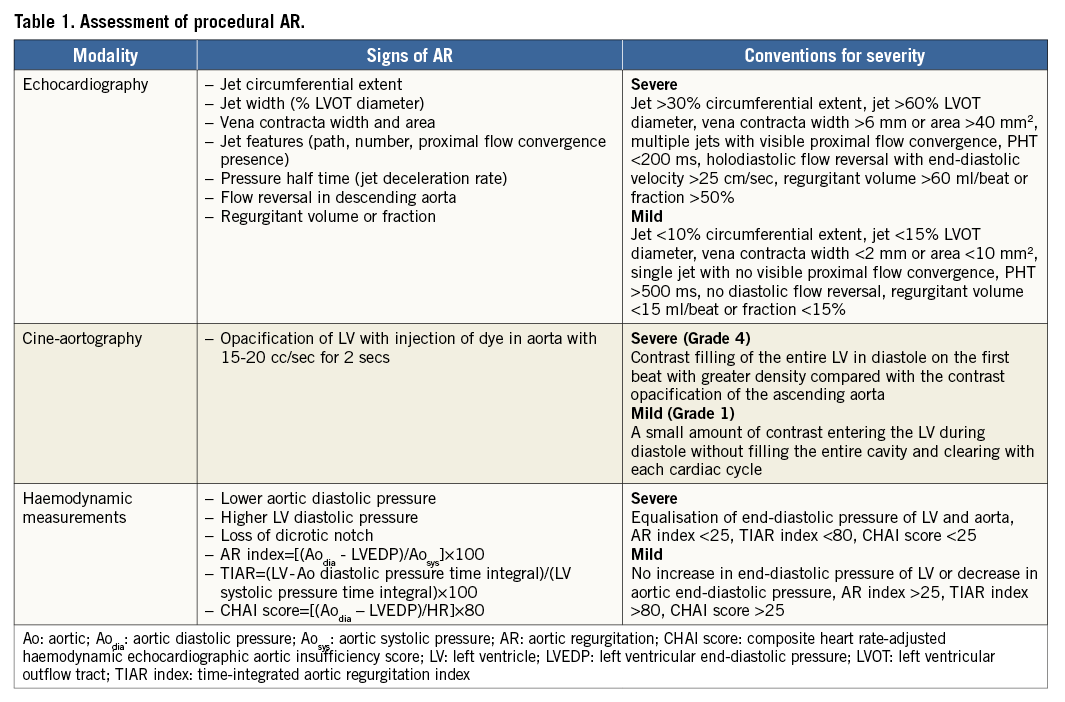
Aortic regurgitation results in acute volume overload to the left ventricle (LV) during diastole. LV pressure response is dependent on the regurgitant volume and diastolic function of the ventricle. Previous studies have demonstrated that LV diastolic dysfunction is common in patients with severe symptomatic AS8. Accurate assessment of LV diastolic pressure can provide useful prognostic information in these patients. While examining the tracing of LV diastolic pressure one should carefully assess end-systolic LV pressure, the rate of rise of LV diastolic pressure and end-diastolic LV pressure. Importantly, the pressure tracing should be compared to pressure tracing prior to TAVI to understand the acute changes better. An increase in LV end-diastolic pressure and an increased rate of rise in LV diastolic pressure are typically seen when AR is severe and leading to LV decompensation. A fall in diastolic pressure and effaced or an absent dicrotic notch indicate significant AR. Integration of some of these measurements has been proposed as an AR index, a simple and practical measure for easy quantification of acute AR9. This index has been validated in TAVI patients. Besides the severity of AR, it is affected by heart rate and underlying LV dysfunction. Modifications of this index have been proposed to minimise interaction with these variables10,11. However, practical implementation of these modifications has been difficult because there is no automated method for computing these more complicated indices.
Aortography with rapid injection of dye is a simple but very useful method for visualising blood flow in the aortic root. In the original description of aortography to determine AR severity, rapid injection of a substantial amount of dye (40 cc of dye injected at 20 cc/sec) was used12. Opacification of the LV in relation to the aorta was proposed as a measure of severity of AR. The exact fluoroscopic projection for this assessment can influence the quantification of AR13. LV opacification appears more intense in LAO projection compared to RAO projection because of foreshortening of the LV cavity in the former. In the current study, the authors used biplanar angiography for AR assessment with rapid injection, which is ideal for angiographic assessment of AR. Angiography also provides critical information regarding location of the prosthesis in relation to the native aortic annulus as well as the coronary arteries. Rare complications such as aortic dissection, annular rupture or coronary occlusion can also be identified with aortography. In patients with acceptable renal function, completion aortography after TAVI is strongly recommended for these reasons.
Echocardiography provides very useful information with regard to the mechanism of AR. As TAVI devices have matured and reduced in profile, several groups proposed a “minimalist” approach where general anaesthesia and TEE are not used during the procedure14. However, TEE is critical for understanding the mechanism of AR. If the patient has renal insufficiency where contrast use needs to be limited, TEE should be strongly considered to assess the severity and mechanism of AR. TTE can be used during TAVI but in many patients image quality proves to be inadequate to exclude any AR with confidence.
In the current report the authors use a systematic strategy to investigate and treat AR after TAVI using angiography, LV pressure measurement and echocardiography. The most important finding is the fact that in almost half (44.7%) of the patients they thought AR was significant enough to warrant a corrective measure. This in itself is an important message. Although the device used is not the most current version of the self-expanding valve, identification of the problem is the first step to a proper solution. Further, they demonstrate that patients with successful correction of the problem had lower one-year mortality compared to patients with persistent residual AR (17.6% versus 50%). They also report that patients with an AR index of >25 had lower mortality compared to those with an AR index <25 (17% versus 34%).
There are important limitations to this study. The investigators used one valve type with one strategy to investigate and minimise AR without any control group to study alternative valves and strategies. Despite their post-implantation interventions, several patients remained with substantial AR. These findings demonstrate the limitations in using precise cut-off values for assessing the severity of AR and related care paths for managing post-TAVI AR. Nevertheless, this report provides a strong argument that one should look with both eyes (echocardiography and angiography) and feel (using pressure measurements) in order to identify AR after TAVI to optimise outcomes.
As we plan to compare different valve types and expand TAVI to a lower-risk population, assessment of AR will play a crucial role in the scientific advancement of the field. Multimodality assessment of AR after TAVI has to be considered as we try to eliminate even mild AR after TAVI to optimise long-term outcomes.
Conflict of interest statement
The authors have no conflicts of interest to declare.

