Abstract
During the last decade, transcatheter aortic valve implantation (TAVI) techniques have evolved rapidly providing several systems that conform to a broad spectrum of aortic valve annulus sizes, developing new delivery systems that provide an alternative to patients with difficult vascular access and permitting more controlled and accurate prosthesis deployment that result in improved procedural outcomes. However, residual aortic regurgitation (AR) (paravalvular or transvalvular) remains a recurrent observation and patients with moderate or severe AR have a reduced mid-term prognosis Therefore, postprocedural AR should be carefully and accurately evaluated in order to decide whether additional procedures such as re-ballooning or valve-in-valve are needed to reduce AR severity, and changes in AR at follow-up should be monitored. In the current review, the role of cardiac imaging to understand the mechanism underlying AR after TAVI and to quantify the severity of AR will be discussed.
Introduction
Transcatheter aortic valve implantation (TAVI) is now an accepted standard of care for patients with symptomatic severe aortic stenosis (AS) who are not candidates for surgery or have high surgical risk. Over the past decade, the combined effect of learning curve, improvement in transcatheter device technology, a better understanding of aortic root anatomy, together with careful patient selection have gradually resulted in an improved clinical outcome for patients undergoing TAVI1. However, aortic regurgitation (AR) remains a common phenomenon following TAVI. Experience in large multicentre studies and registries, including over 6,000 patients, revealed that the incidence of AR after TAVI ranged from 48 to 93% of patients, with comparable prevalence between self- and balloon-expandable prostheses (Table 1)2-10. Trace or mild paravalvular AR is a common finding in the majority of patients, whereas only 14-21% of patients may have at least moderate AR following TAVI (Table 1)2-10. The observed variation in AR after TAVI may be related to the use of different modalities to assess AR (angiography, transoesophageal [TEE] or transthoracic [TTE] echocardiography), the different timing of AR assessment after TAVI (immediately post deployment, before hospital discharge and at 30-day follow-up) and the lack of a standardised protocol to grade AR severity (qualitative versus semi-quantitative methods). Accurate assessment of AR after TAVI is clinically relevant since moderate and severe post-procedural AR have been associated with poor treatment response11, early in-hospital death2 and mid-term mortality3,5,8,11. In addition, a recent report has suggested that residual AR grade >1 may also be associated with reduced survival12. Conversely, serial evaluations have shown that the severity of AR tends to reduce over time3,13,14. Current recommendations to characterise and quantify the severity of AR post TAVI include the morphology and location of the regurgitant jet, as well as the percentage of the circumference of the prosthesis occupied by the regurgitant jet. The value of cardiac imaging to evaluate the mechanisms underlying AR after TAVI and to quantify its severity will be discussed in this review.
Mechanisms of AR after TAVI
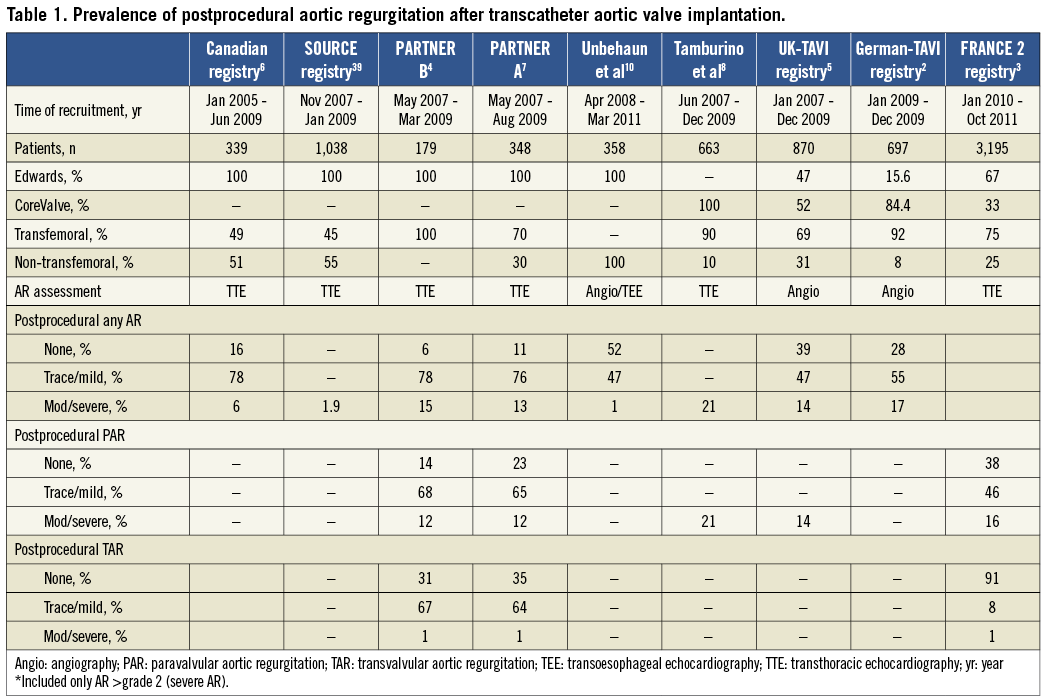
AR following TAVI can be categorised according to the location of the AR jet in relation to the prosthesis: either paravalvular AR (PAR) (between the prosthesis and the native annulus), transvalvular AR (TAR) (within the prosthesis) or both, and is best appreciated using colour-flow Doppler echocardiography (Figure 1).
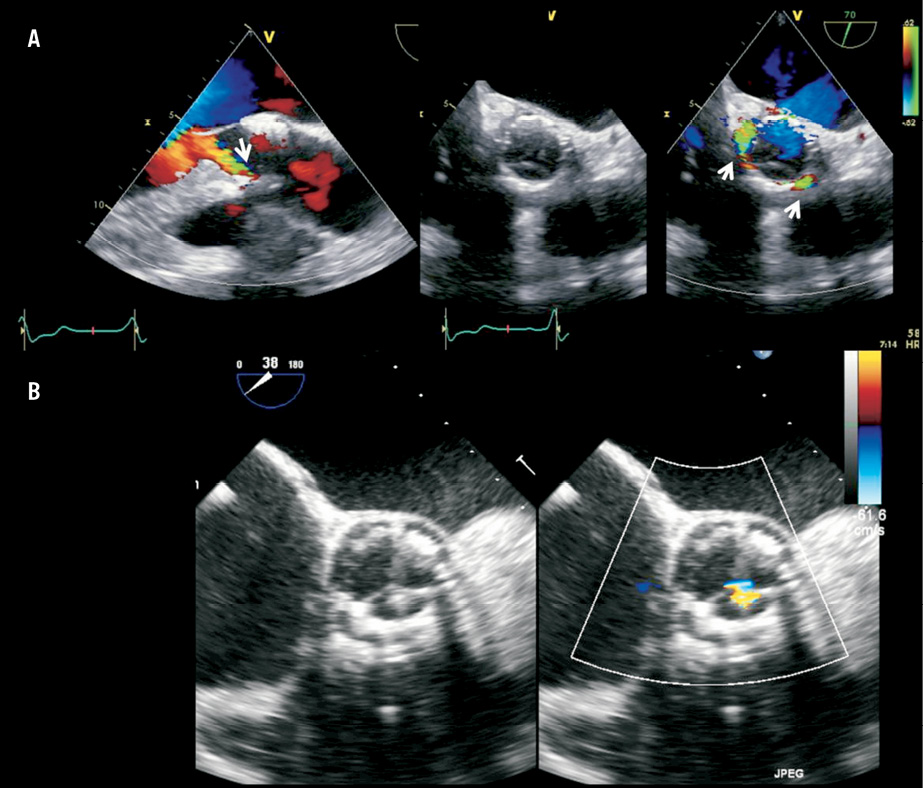
Figure 1. Panel A shows the long-axis view of the aortic root on conventional 2-D transoesophageal echocardiography of a patient who underwent transcatheter aortic valve implantation with a balloon-expandable valve. The arrow indicates the presence of mild paravalvular regurgitation at the level of the anterior part of the aortic annulus. The short-axis views show the exact location of the two jets of paravalvular regurgitation (arrows). Panel B shows a patient with transvalvular regurgitation, best noted on the short-axis view on transoesophageal echocardiography.
PAR can be due to incomplete annular sealing of the transcatheter valve. With conventional aortic valve surgery the native aortic valve is removed and the prosthesis is sewn onto the decalcified annulus, but in TAVI the native calcified aortic leaflets are displaced to accommodate the newly implanted prosthesis. The presence of asymmetrical/bulky aortic valve calcification has been related to the presence of PAR (Figure 2)10,15-18. Exact localisation of calcium in the aortic root and valve may predict PAR after TAVI19,20. Particularly, calcification of the commissures has been associated with PAR but calcification of the body or the edge of the aortic cusps did not increase the risk of PAR20,21. Other phenomena related to PAR include prosthesis undersizing18,22,23, bicuspid valve (asymmetrical expansion)24, incorrect depth of implantation (too high or low implantation without apposition to annular tissue) and an increased angle of the left ventricular outflow tract (LVOT) to the ascending aorta25. Multidetector row computed tomography is a valuable preprocedural imaging technique providing accurate sizing of the aortic annulus, precise characterisation of aortic valve anatomy and aortic root dimensions, and localisation of calcifications in the landing zone (Figure 2)20,22,23.
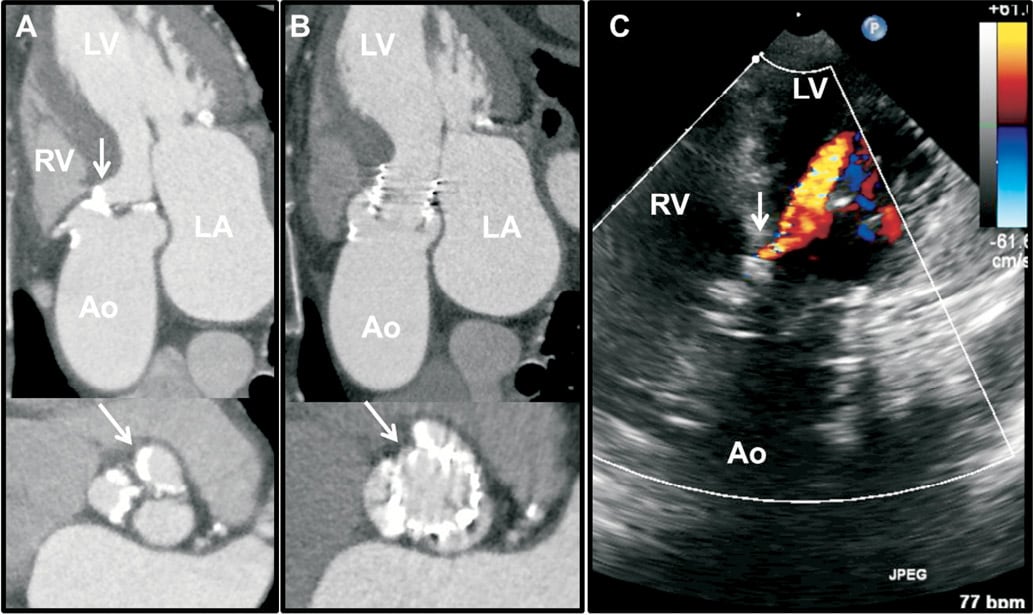
Figure 2. Panel A shows a calcified tricuspid aortic valve of a patient with bulky calcification mainly in the non-coronary (arrow) and right cusps, and extending to the base of the interventricular septum (arrow) on multidetector computed tomography (MDCT). Following TAVI with a balloon-expandable (SAPIEN XT) valve, a paravalvular leak was observed with colour-coded Doppler transoesophageal echocardiography (TEE) that coincided with the location of the bulky calcification at the commissure between the right and non-coronary cusps on MDCT. Panel B shows the deployed frame and the arrow indicates the gap at the level of the commissure between the non-coronary and right cusps. Panel C shows the colour-coded Doppler TEE in a transgastric view with the paravalvular leak in the location of the calcification at right to non-coronary commissure.
In contrast, TAR is less often observed and may arise from valvular obstruction (from stiff guidewire or pigtail catheter or an overhanging native leaflet resulting in improper leaflet closure)26,27, valvular damage (during crimping process or overexpansion following post-dilatation)26,28 or prosthesis oversizing (suboptimal stent expansion or impaired leaflet mobility). Prompt recognition of TAR and its mechanism is crucial, so that appropriate interventions such as manipulation of catheter or implantation of a second prosthesis (valve-in-valve procedure) can be performed in a timely manner to ensure good clinical outcome.
Imaging and quantification AR after TAVI
Currently, AR after TAVI is largely assessed using angiography, echocardiography or both. Using supra-aortic angiography, the degree of postprocedural AR is determined qualitatively by visual estimation of the concentration of contrast medium (regurgitation volume) in the left ventricle (LV) and classified into four grades: absent (grade 0), trace or mild (grade 1), mild-to-moderate (grade 2), moderate-to-severe (grade 3) and severe (grade 4)29. Although this is a commonly used method of assessment during TAVI, it lacks accurate quantification and has limited sensitivity in differentiating PAR from TAR (Figure 3). Moreover, this modality is not preferred for serial AR evaluation after TAVI.
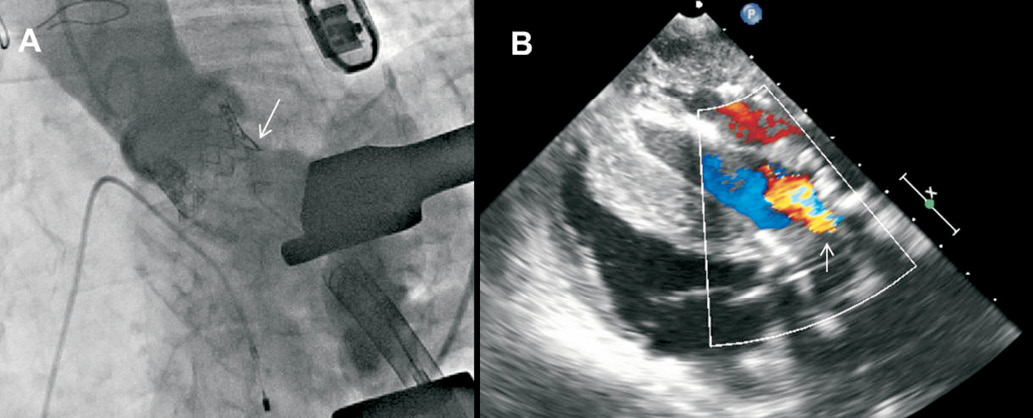
Figure 3. Assessment of aortic regurgitation (AR) after TAVI. Panel A shows supra-aortic angiography to estimate the AR grade. In contrast to echocardiography, angiography has limited resolution to differentiate between paravalvular and transvalvular AR. Panel B shows the TEE 120º transgastric view of the same patient. The arrow indicates the presence of a wide regurgitant jet in the valve due to a frozen cusp.
The other widely used method for AR assessment is echocardiography, and TEE is frequently performed to help guide transcatheter valve deployment and to detect complications during TAVI30. In addition, it permits assessment of the position and function of the prosthesis, including determination of the presence/severity and mechanism of AR immediately after valve deployment. In the setting of TAVI, it is critical to distinguish PAR from TAR and to determine its severity rapidly so as to allow for manoeuvres such as re-ballooning, attempting maximal expansion of the prosthesis in the presence of significant PAR or deployment of a second valve in the presence of severe TAR. Using the standard long- and short-axis views of colour Doppler TEE, PAR and TAR can both be accurately detected. With the current three-dimensional matrix array TEE probes, simultaneous display of two orthogonal real-time images (biplane long- and short-axis views) with superimposed colour flow Doppler imaging allows further delineation and exact localisation of the PAR (Figure 4). However, accurate quantification of PAR remains challenging as it frequently consists of multiple small jets, origins of which may be obscured by the prosthesis stent/frame. Current recommendations for AR assessment after TAVI are derived from native valvular regurgitation, using multi-parametric approaches (Table 2)31,32. In the setting of TAVI, modifications are required as the grading for PAR differs from that of TAR, with emphasis on the “jet anatomy” classification. For example, the width of the proximal AR jet relative to the LVOT diameter is the suggested criterion for semi-quantitative assessment of TAR severity (Figure 5). In contrast, as PAR is frequently eccentric and irregular in shape, the proportion of the circumference of the prosthesis covered by the AR jet provides semi-quantitative assessment of PAR severity. However, this approach does not take into consideration the presence of multiple jets (unknown validity of summation of all the jets) and the possible contamination from the radial extent of PAR jets, which may result in overestimation of the AR severity32. Finally, with the currently available 3-D colour full volume echocardiographic data sets, direct planimetry of the AR vena contracta may provide an accurate quantitative assessment of AR severity (Figure 5). Recently, Goncalves and co-workers showed that quantitative assessment of PAR after TAVI was feasible by planimetry of the vena contracta obtained with 3-D TTE33. The 3-D approach provided a better correlation between the AR volume and the vena contracta as compared to 2-D TTE.
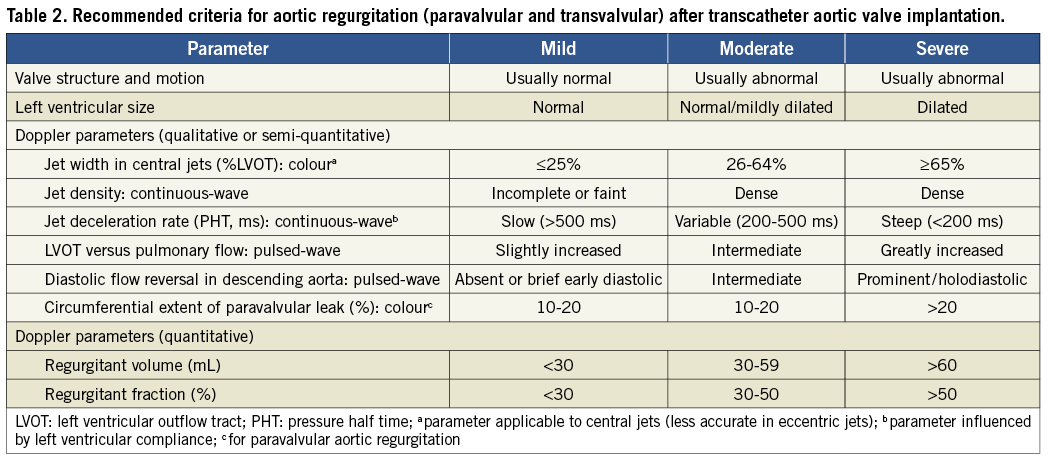
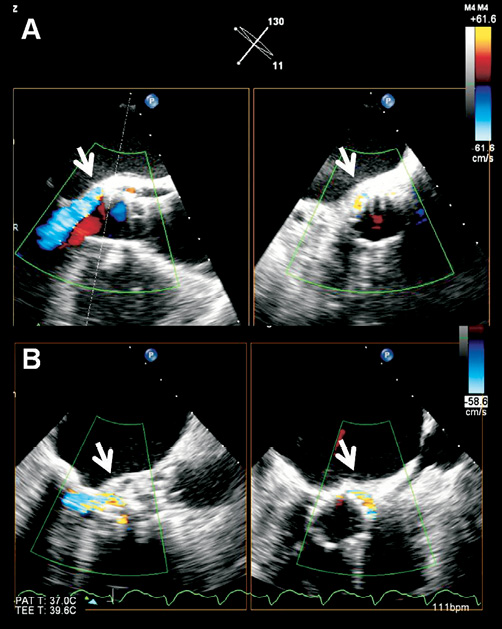
Figure 4. Current 3-D transoesophageal probes permit simultaneous visualisation of the transcatheter aortic valve in orthogonal (long- and short-axis) views to exactly localise the paravalvular leak. The arrows indicate that the paravalvular leak is located at the posterior part of the aortic annulus, close to the anterior mitral valve leaflet. The paravalvular leak is mild in severity as it occupies <10% of the circumference of the prosthetic valve (A) and is moderate in severity as it occupies 10-20% of the circumference of the prosthetic valve (B).
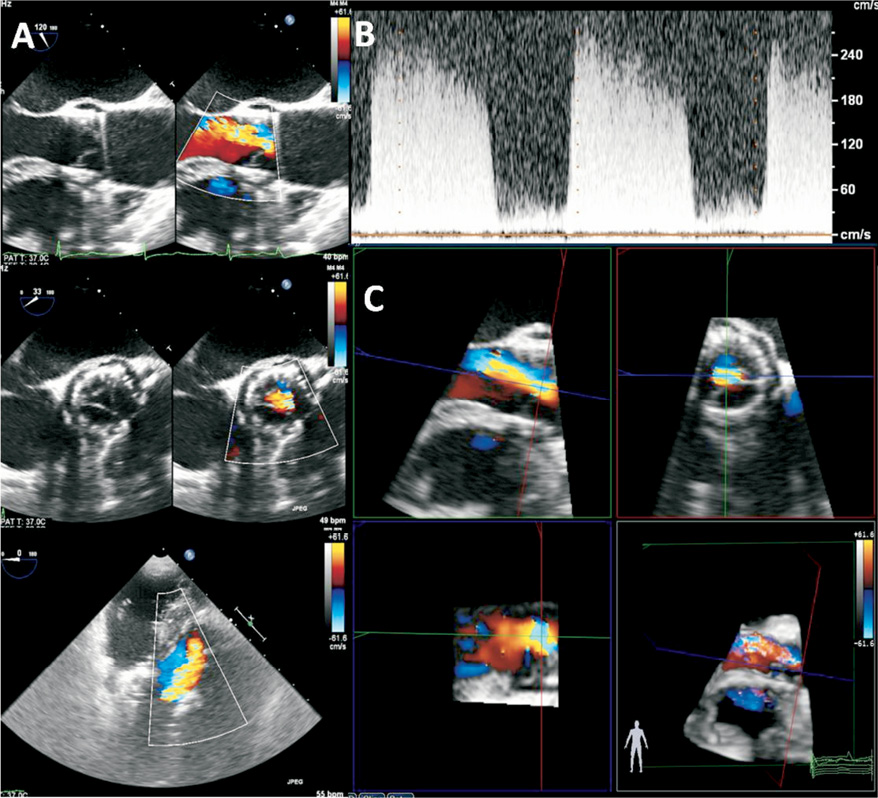
Figure 5. An example of moderate-to-severe transvalvular aortic regurgitation (AR) after TAVI, assessed semi-quantitatively using Doppler parameters: a jet width that occupied 45% of the LVOT diameter (A), a dense CW Doppler signal and a steep jet deceleration rate (B). 3-dimensional colour-coded Doppler permits quantitative evaluation of AR using multiplanar reconstruction by planimetry of the regurgitant vena contracta area (C), which is performed by selecting the best frame to visualise the AR jet. The 3-D dataset was manually cropped to provide a cross-sectional plane through the vena contracta of the AR jet, perpendicular to the direction of the jet. From the en face view of the vena contracta and selecting the plane with the narrowest cross-sectional area of the regurgitant jet, planimetry of the vena contracta can be performed.
Localisation of the PAR based on the standard parasternal short-axis TTE view of the aortic prosthesis is possible. In addition to a direct measurement of the vena contracta33, 3-D TTE may provide a more accurate calculation of the total stroke volume (both the regurgitant and forward stroke volumes) by subtracting LV end-systolic from LV end-diastolic volumes34. In the absence of significant mitral regurgitation, this method may be highly accurate since it is independent of geometric assumptions and is not hampered by foreshortened views. Therefore, 3-D echocardiography may become the method of choice for assessing complicated AR such as that following TAVI, although it requires further validation before widespread implementation in clinical routine.
The feasibility of magnetic resonance imaging (MRI) for quantification of AR post TAVI has been evaluated: particularly velocity-encoded phase imaging permits measurement of blood flow velocity and volume across the valve and calculation of the regurgitant fraction (ratio of forward to backflow volume across the valve). A recent study including 16 patients who underwent MRI following TAVI demonstrated a significant correlation between MRI-derived and angiography-estimated degree of AR (r=0.86, p<0.001) while only a limited correlation between MRI and 2-D TTE was observed (r=0.32, p=0.23)35. Moreover, TTE underestimated AR by at least one grade when compared to MRI in 44% of patients, indicating the limitations of 2-D imaging for assessment of eccentric AR, in particular PAR post-TAVI35.
Experience of AR assessment after TAVI and when to measure
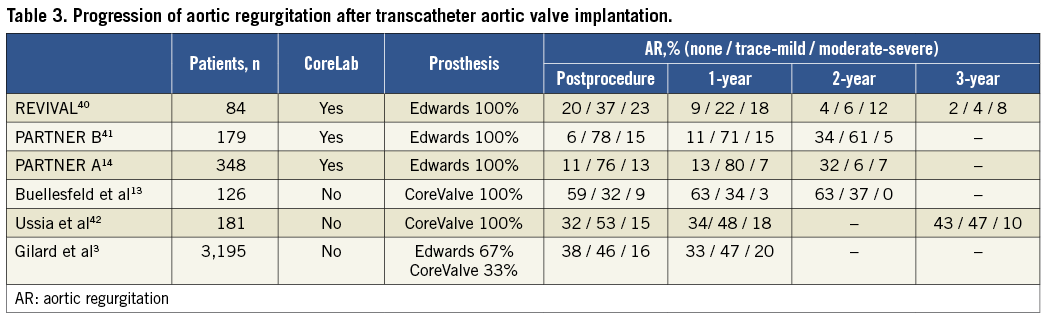
Acute postprocedural evaluation of AR after TAVI is crucial since the presence of moderate and severe AR is associated with increased mortality at follow-up and several manoeuvres can be performed at the catheterisation laboratory/hybrid operating theatre to reduce the severity of AR. In addition, the presence of AR should be monitored during the days after TAVI since the regurgitation grade may change (Table 3). For example, the properties of the self-expandable prosthesis may lead to a reduction in the grade of PAR at follow-up. In 126 patients undergoing TAVI with self-expandable prostheses, Buellesfeld et al reported a reduction in the prevalence of PAR of any grade from 41% at 30 days after TAVI to 37% at two-year follow-up13. Experience with the balloon-expandable prostheses has also shown a progressive reduction in the prevalence of AR after TAVI. In the PARTNER cohort A trial, AR improved in 31.5% of patients at two-year follow-up14. Conversely, in the FRANCE 2 registry with 3,195 patients, the prevalence of AR remained unchanged at one-year follow-up3.
Anatomo-pathological analyses of explanted self-expandable prostheses have demonstrated neointimal coverage of the frame struts in contact with the aortic wall but not in areas of high velocity blood flow such as the coronary ostia36. This neointimal tissue may be beneficial in reducing the grade of PAR by closing the gaps between the prosthetic frame and the native annulus. However, it has been suggested that, in specific circumstances, this tissue proliferation may lead to more rapid structural valve deterioration. So far, structural valve deterioration is anecdotal37,38 and other complications such as stent fracture, deformation or valve migration have not been described.
Conclusions
AR after TAVI has been associated with worse outcome and increased mortality at follow-up. Accurate assessment of AR during the procedure is crucial to decide whether additional manoeuvres such as re-ballooning or valve-in-valve are needed to reduce the AR grade. Supra-aortic angiography or echocardiography (particularly 3-D TEE) are the preferred imaging techniques to assess AR immediately after valve deployment. In addition, continued evaluation of AR at follow-up is recommended since the grade of AR may change over time. For follow-up assessment, transthoracic echocardiography, particularly 3-D TTE, is the preferred imaging technique.
Conflict of interest statement
V. Delgado received consulting fees from Medtronic and St. Jude Medical. The Department of Cardiology received research grants from Lantheus Medical Imaging, Boston Scientific, Sadra Lotus, Medtronic, St. Jude Medical, Biotronik, Edwards Lifesciences and GE Healthcare. The other authors have no conflicts of interest to declare.

