Abstract
Significant aortic regurgitation (AR) after transcatheter aortic valve implantation (TAVI) has been shown to be associated with worse mid-term outcome. The two-year follow-up results of the PARTNER US trial showed that not only ≥3/4 AR grade, but also grade 2 had a significant impact on mortality. Thus, prevention and treatment of significant AR after TAVI is of great importance. Usually, AR after TAVI consists mostly of paravalvular leak and significant central AR is uncommon. Here we describe measures to decrease the risk of AR after TAVI which are currently available.
Introduction
Significant aortic regurgitation (AR) after transcatheter aortic valve implantation (TAVI) was identified as an independent predictor of in-hospital mortality in the German registry study1. More recently, it has also been shown to be associated with late mortality in the Italian2 and the French registries3. Furthermore, the recently published two-year follow-up results of the cohort A of the PARTNER US trial showed that not only AR grade 3 or 4, but also mild AR of grade 2/4, had a significant impact on the long-term outcome4. Data from our prospective registry have shown similar findings (Figure 1) and clearly a worse mid-term outcome in cases of AR grade 2 post TAVI (compared to grade 0 or 1), not only with the CoreValve® (Medtronic, Inc., Minneapolis, MN, USA) but also with the Edwards valve (Edwards Lifesciences, Irvine, CA, USA) (Figure 2)5. Thus, prevention, evaluation and treatment of AR after TAVI seem to be of great importance, even for mild AR.
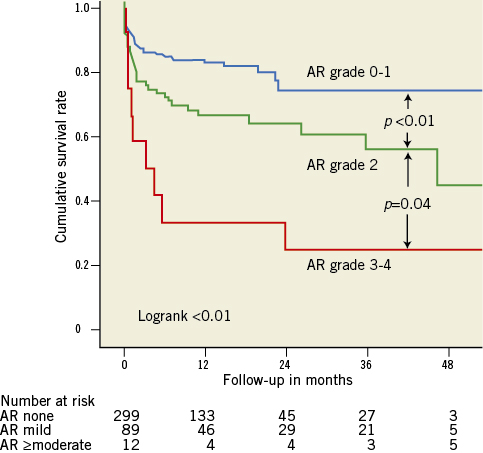
Figure 1. Survival curves by post-procedural AR. Survival curves for post-procedural AR grade: 0-1 (blue), 2 (green) and 3-4 (red) (Massy data). Cumulative survival rates calculated by the Kaplan-Meier method and compared with the log-rank test.
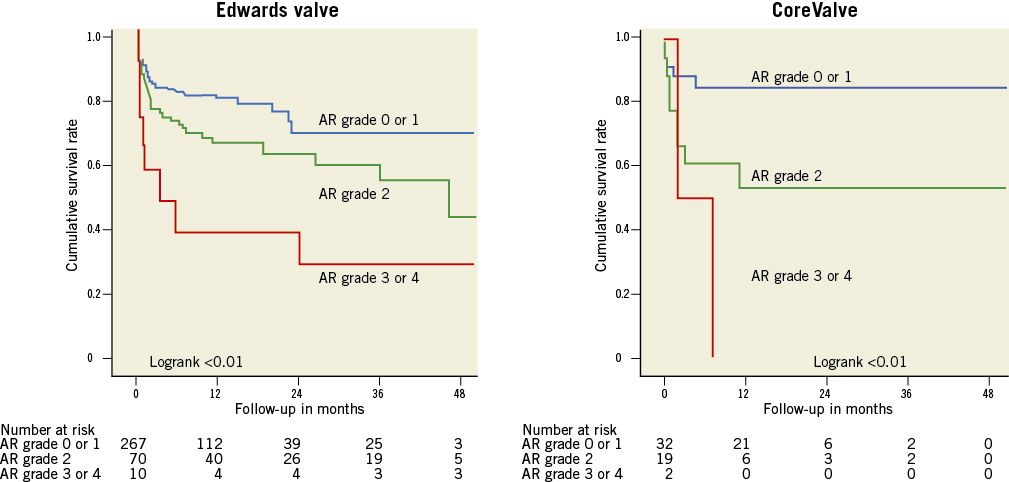
Figure 2. Survival curves by post-procedural AR. Survival curves for post-procedural AR grade: 0-1 (blue), 2 (green) and 3-4 (red) (Massy data). Cumulative survival rates calculated by the Kaplan-Meier method and compared with the log-rank test.
Figure 2. Survival curves by post-procedural AR. Survival curves for post-procedural AR grade: 0-1 (blue), 2 (green) and 3-4 (red) (Massy data). Cumulative survival rates calculated by the Kaplan-Meier method and compared with the log-rank test.
Mechanisms of AR after TAVI
Generally, AR after TAVI mostly consists of paravalvular leak and, although significant, central AR is an uncommon occurrence which should be identified immediately.
In cases of a moderate to severe central leak, an attempt at mobilisation of the failing valve has been shown to be successful in some instances6. In cases of failure, the decision to perform a valve-in-valve procedure should be taken promptly.
The causes of paravalvular leak are multifactorial7. The main mechanism of AR results from valve undersizing due to underestimation of the annulus size or incomplete inflation of the Edwards valve delivery balloon. A decrease in the degree of paravalvular leak can be observed later with the CoreValve due to its self-expanding nature.
The presence of calcification at the level of the annulus or the commissures may also play an important role8,9 (Figure 3). Though a less frequent cause, implantation of the valve in a position either too low or too high can lead to AR. In recipients of a CoreValve, if the aortic annulus plane is 12 mm higher than the lower end of the metal frame, the gap between the annulus plane and the skirt will cause a severe paravalvular leak (Figure 4). The optimal landing zone and skirt height for each valve is summarised in Figure 5 and Figure 6.
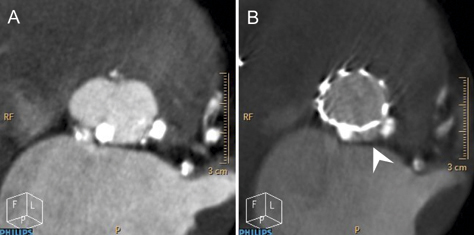
Figure 3. Huge (very large) calcified nodule in the aortic annulus. A) Two calcified nodules in the aortic annulus. B) A gap between two calcified nodules (arrow head) may cause moderate aortic regurgitation.
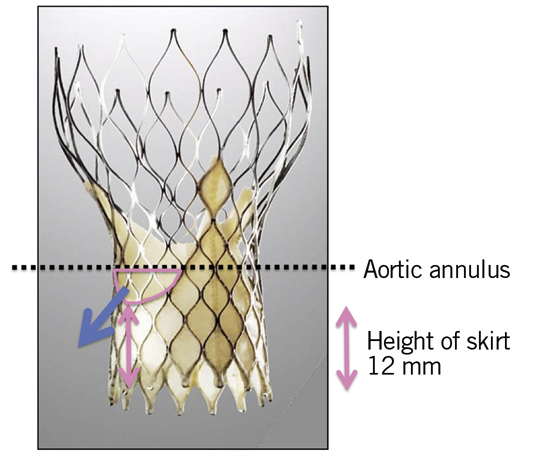
Figure 4. Mechanism of AR in cases of too low implantation of a CoreValve. Pink double-sided arrow: the height of the lower part of a valve (12 mm); blue arrow: aortic regurgitation; black lines: aortic annulus plane.
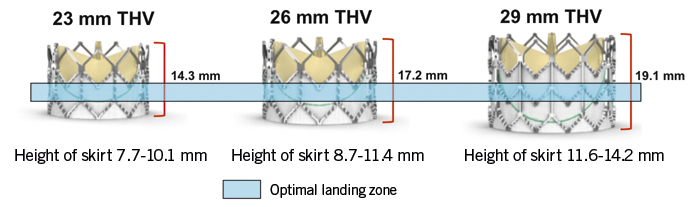
Figure 5. Skirt height and optimal landing zone for the Edwards valve 23, 26 and 29 mm.
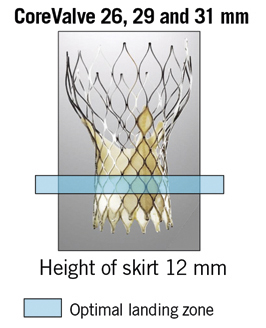
Figure 6. Skirt height and optimal landing zone for the CoreValve 26, 29 and 31 mm.
Despite the paucity of data about late onset AR, this can be the result of central regurgitation rather than paravalvular leak and a valve-in-valve strategy may solve this issue.
Treatment of AR after TAVI
The objective in terms of paravalvular leak after TAVI is to obtain an AR
Post-dilatation
Balloon post-dilatation is the first option for the treatment of significant paravalvular leak after TAVI when the valve is in a landing zone. In recent studies it has been shown to be used in 10-28%8,9 of patients after Edwards valve implantation. Post-dilatation with a larger balloon may effectively reduce the grade of paravalvular leak in the majority of cases8,9 (Figure 7). However, excessive balloon oversizing should be avoided because post-dilatation carries an inherent risk of annulus rupture, incomplete coaptation of the bioprosthetic leaflets and accelerated bioprosthesis degeneration. In our centre, post-dilatation is performed using the balloon delivery system with an additional 1 to 3 ml of contrast mix for the Edwards valve; for the CoreValve, a balloon diameter equal to the aortic annulus size is used. When post-dilatation is performed, the use of rapid pacing is crucial and pacing failure of even a single beat may result in valve migration during post-dilatation and subsequent valve embolisation (Figure 8). In a recently published single-centre series of 211 patients treated with the Edwards valve8, post-dilatation was associated with a significant increase in valve diameter and a significant reduction in AR in 72% of cases. Post-dilatation was not associated with any deleterious effect on valve function or mortality at mid-term follow-up, but a higher rate of cerebrovascular events was observed, which may limit the technique. Further studies are necessary to elucidate this cerebrovascular issue, which was not observed in our experience.
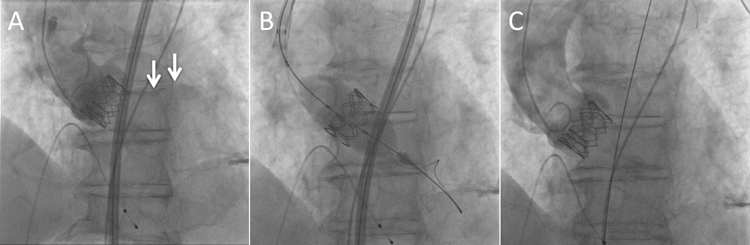
Figure 7. Improvement of paravalvular leak after post-dilatation. A) Severe paravalvular leak after deployment of a balloon-expandable valve. B) Post-dilatation with additional 1.5 ml in the balloon. C) Improvement of paravalvular leak.
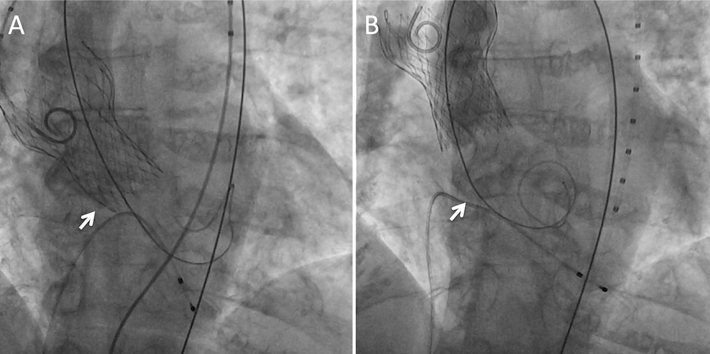
Figure 8. Pacing failure caused migration of a self-expandable valve during post-dilatation. Severe AR after deployment of a 31 mm self-expandable valve to a large annulus. Arrow: annular level. One beat of pacing failure caused migration of the valve into the ascending aorta. Arrow: annular level.
Valve mobilisation with the CoreValve
In some cases, aortic regurgitation is due to positioning the valve too low. Pulling back the valve with one or two snare loops will solve the problem in the majority of cases.
Valve-in-valve
Deployment of a second bioprosthesis in the first one (valve-in-valve) can be effective for AR after TAVI caused by central leak or by certain types of paravalvular leak11,12. In a series of 760 TAVI cases reported recently by Toggweiller et al, there were 21 valve-in-valve cases using the Edwards valve, due to central leak in three cases and paravalvular in 18 cases, with a 90% success rate13. A case example of valve-in-valve for severe AR is shown in Figure 9. Implantation of a larger second valve bioprosthesis in a lower position than the first one in order to seal the gap between the bioprosthesis and the annulus may be effective for paravalvular AR refractory to post-dilatation. Moreover, this procedure is the only solution for a self-expandable valve which is implanted in a very low position and causes a leak through the frame struts. In cases of central leaks, the second valve can be the same size as the previously implanted one or can be larger if the problem is related to annulus underestimation.
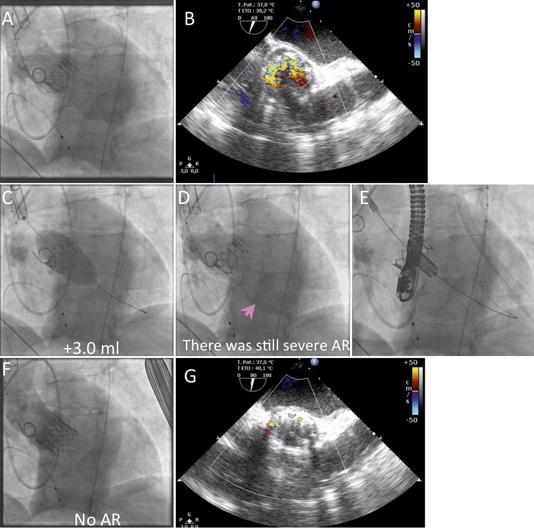
Figure 9. Successful management of severe paravalvular leak by implantation of a second balloon-expandable valve (valve-in-valve). A) Severe paravalvular leak after implantation of a balloon-expandable valve. B) Echocardiography showed severe paravalvular leak. C) Post-dilatation with additional 1.5 ml and 3.0 ml was performed. D) No improvement of paravalvular leak was observed. E) Deployment of the second valve in the first one. F) Disappearance of paravalvular leak. G) Trivial paravalvular leak on echocardiography.
Percutaneous closure of paravalvular leak
Percutaneous closure using a specific occluder (e.g., AMPLATZER™ Vascular Plug [St. Jude Medical, St. Paul, MN, USA] etc.) can be applied to paravalvular leak after TAVI and some case reports have recently been presented. However, the indication of this procedure is still the subject of debate as it may cause valve embolisation.
Surgical correction
This strategy is considered when post-procedural AR is grade 3 or 4 and refractory to other solutions. However, a TAVI cohort is usually at high perioperative risk or inoperable; therefore, this treatment should be thoroughly discussed, and implemented as a last resort.
New technologies
The second generation of the Edwards valve, the SAPIEN 3 valve (Edwards Lifesciences, Irvine, CA, USA) (Figure 10A) has added a special skirt to reduce the risk of paravalvular leak, which may decrease the need for post-dilatation. Newcomers such as Symetis (Symetis SA, Ecublens, Switzerland) (Figure 10B), Portico (St. Jude Medical, Minneapolis, MN, USA) (Figure 10C), Sadra Lotus™ valve (Boston Scientific, Natick, MA, USA) (Figure 10D) which are self-expandable valves also have a kind of skirt-like structure to minimise the risk of paravalvular leak. Another newcomer, the Direct Flow Medical valve (Direct Flow Medical, Inc., Santa Rosa, CA, USA) (Figure 10E) has a conformable ring which covers the left ventricular outflow tract side of the aortic valve to minimise paravalvular leak. These new valves are also repositionable, which may prove useful if the valve is too small, too low or too high.
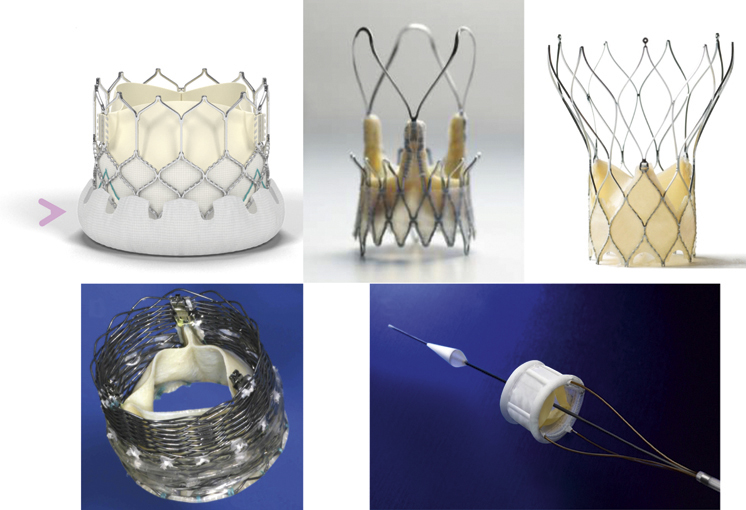
Figure 10. New generation of valves.
Conclusions
The best treatment for AR is prevention, by optimal and meticulous valve assessment and annulus sizing, valve positioning and deployment. In cases of significant AR, the underlying mechanism should be clearly identified by echocardiography. When AR is paravalvular and due to valve undersizing, post-dilatation has been shown to be effective in many cases. When the mismatch is too high or the valve is too high or too low, implementation of a valve-in-valve technique is probably the best option in these high-risk patients.
Acknowledgements
The authors would like to thank Mrs Catherine Dupic for her assistance in the preparation of this manuscript.
Conflict of interest statement
T. Lefèvre is proctor for transfemoral-TAVI for Edwards Lifesciences, and is consultant for Symetis and Direct Flow Medical. K. Hayashida has no conflict of interest to declare.

