Abstract
Aims: Residual paravalvular aortic regurgitation (PAR) after transcatheter aortic valve implantation (TAVI) is common. We therefore evaluated incidence, determinants and outcome of PAR after TAVI.
Methods and results: Data from 167 consecutive transcatheter TAVI patients were analysed. PAR was graded by angiography and the pressure gradient between diastolic aortic pressure and left ventricular end-diastolic pressure (∆PDAP-LVEDP) after implantation. TAVI was technically successful in all patients. Mortality was 9% and 20% at 30 days and one year, respectively. Post-procedural PAR was absent in 54 patients (32.3%). Mild PAR was found in 89 (53.3%), moderate in 21 (12.6%), and moderate-to-severe in three patients (1.8%). Cardiovascular mortality at 30 days and one year was increased in patients with moderate and moderate-to-severe PAR compared to patients with no and mild PAR (46% vs. 4% and 73% vs. 7%, respectively, p<0.001). Receiver operating characteristic curve analysis suggested ∆PDAP-LVEDP ≤18 mmHg as a novel predictor of mortality, with an area under the curve of 0.97.
Conclusions: In patients undergoing TAVI, moderate and moderate-to-severe PAR was observed in 14.4% and associated with increased cardiovascular mortality. A pressure gradient ∆PDAP-LVEDP≤18 mmHg carries adverse prognosis and requires further intervention.
Abbreviations
DAP/ diastolic aortic pressure
∆PDAP-LVEDP/ pressure gradient between DAP and LVEDP
ES/ Edwards SAPIEN (XT)
LVEDP/ left ventricular end-diastolic pressure
LVEF/ left ventricular ejection fraction
LV/ left ventricular, left ventricle
MCV/ Medtronic CoreValve
PAR/ paravalvular aortic regurgitation
AVR/ surgical aortic valve replacement
TAVI/ transcatheter aortic valve implantation
TEE/ transoesophageal echocardiography
Introduction
Paravalvular aortic regurgitation (PAR) is considered a typical complication after surgical aortic valve replacement (SAVR)1. More than mild PAR occurs in only 2% of SAVR, but is associated with an increased haemodynamic burden and often requires reintervention2,3. The incidence of moderate or severe PAR after transcatheter aortic valve implantation (TAVI) is higher and varies from 10% to 20%2,4-7. The TAVI operator is then faced with the problem as to whether or not PAR should be corrected immediately, e.g., by post-dilatation or valve-in-valve implantation8.
There is growing evidence that moderate-to-severe PAR after TAVI is associated with increased in-hospital mortality9 and unfavourable long-term outcome5,10. Precise judgement of PAR severity is a prerequisite of treating it effectively and of improving survival10,11. PAR severity during TAVI is usually graded qualitatively using angiography. Recently, quantitative haemodynamic grading with an aortic regurgitation index has been proposed: this is calculated as the ratio of the gradient between diastolic blood pressure and left ventricular end-diastolic pressure (LVEDP) to systolic blood pressure. This index predicts one-year mortality after TAVI11. A simpler and more readily available index to guide peri-interventional clinical decisions in TAVI patients would be even more useful. Therefore, the purpose of the present study was to characterise systematically the incidence, severity, determinants and outcome of residual PAR after TAVI and to assess its severity quantitatively from the transaortic pressure gradient in order to facilitate on-table decisions.
Methods
PATIENT POPULATION
Between November 2006 and December 2010, 167 consecutive high-risk patients with symptomatic aortic valve stenosis underwent TAVI using the Medtronic CoreValve (MCV) (Medtronic Inc., Minneapolis, MN, USA; n=88 [52.7%]) and the Edwards SAPIEN (ES), (Edwards Lifesciences Inc., Irvine, CA, USA; n= 79 [47.3%]) bioprostheses. The decision for TAVI was made by an interdisciplinary heart team and based on current recommendations5-7,9. TAVI procedures were performed according to previously reported standard techniques6,7,12. Cardiovascular mortality was used as an endpoint according to Valve Academic Research Consortium (VARC) recommendations12.
RESIDUAL PARAVALVULAR AORTIC REGURGITATION
Residual PAR was graded using angiography, and transaortic pressure measurements were made after final device deployment and removal of the catheter and guidewire. Angiography was performed perpendicular to the native valve plane in a slight cranial/LAO projection over a 6 Fr pigtail catheter (Cordis Corporation, East Bridgewater, NJ, USA) placed 2-4 cm above the aortic annulus. PAR was graded qualitatively from contrast reflux using the Sellers method9,13,14 after injecting 40 cc of contrast with a flow rate of 20 cc/s: grade 0 was defined as no PAR. Mild (1/4) PAR was defined by confinement of the jet to the left ventricular (LV) outflow tract and disappearance at each systole. PAR was graded moderate (2/4) when the contrast jet remained in the LV for more than one systolic contraction. Continuous LV filling over 2-3 cardiac cycles was graded as moderate-to-severe (3/4), and PAR was considered severe (4/4) when the entire LV opacified at the first cardiac cycle. For the present study, residual PAR was considered relevant when ≥2/4.
The pressure gradient between diastolic aortic and LV end-diastolic pressure (∆PDAP-LVEDP) was measured using a 6 Fr pigtail catheter in the midventricular LV and the pigtail catheter used for the aortogram (Figure 1). Simultaneous pressures were recorded at 50 mm/s and averaged over three representative cardiac cycles for both sinus rhythm and atrial fibrillation. Since transfemoral TAVI was performed under conscious sedation, transoesophageal echocardiography (TEE) was not routinely used, but was available as a stand-by in case of complications.
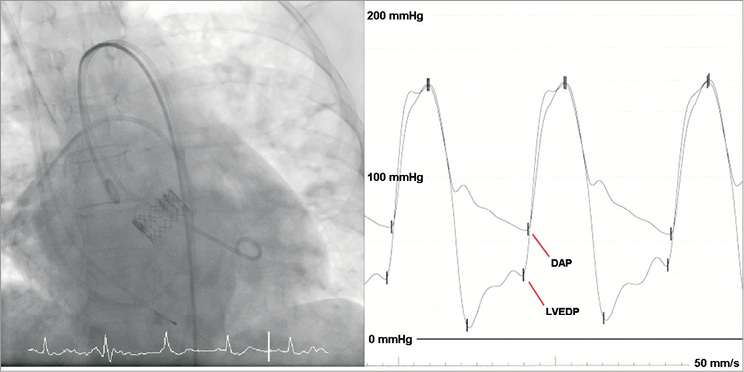
Figure 1. Invasive haemodynamics. Simultaneous measurement of aortic and left ventricular pressure at the end of the TAVI procedure using a 6 Fr pigtail catheter positioned in the midventricular LV and a pigtail catheter placed 2-4 cm above the aortic annulus.
STATISTICAL ANALYSIS
Categorical data are presented as frequencies and percentages; continuous variables are expressed as mean and standard deviation. Comparisons were made with two-sided χ²-tests or two-sided Fisher’s exact tests for categorical variables and one-way ANOVA for continuous variables, using Bonferroni correction for multiple testing. ANOVA was used for comparing more than two groups and t-test or Mann-Whitney for two-group comparison. A p-value <0.05 was considered significant. Survival analysis was performed by the Kaplan-Meier method for PAR grading according to Sellers and ∆PDAP-LVEDP, with patients censored as of the last date they were known to be alive. Subgroup analysis was performed by quartiles of ∆PDAP-LVEDP. Receiver operating characteristic curve analysis was performed for ∆PDAP-LVEDP as a potential predictor of cardiovascular mortality in the setting of PAR.
Multivariate logistic regression was used to define predictors of PAR and cardiovascular mortality from PAR. Variables were entered into the multivariate models if univariate analysis was marginally suggestive of unadjusted association with relevant PAR (p-value <0.2) or if deemed of clinical importance. All statistical analyses were performed using SPSS (version 17.0; SPSS, Chicago, IL, USA). The authors had full access to the data and take full responsibility for their integrity. All authors have read and agree to the manuscript as written.
Results
PAR FOLLOWING TAVI: PATIENT AND PROCEDURAL CHARACTERISTICS
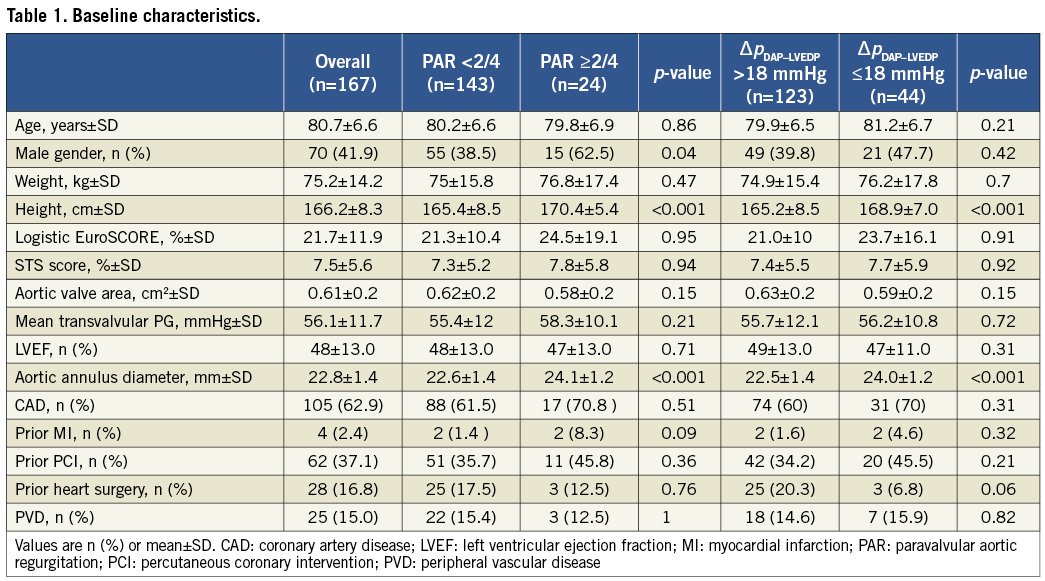
Our study cohort represents a typical TAVI patient population of elderly (80.7±6.6 years) patients with symptomatic aortic stenosis (aortic valve area 0.61±0.3 cm², transvalvular gradient 56.1±11.7 mmHg) at high operative risk (logistic EuroSCORE of 21.7±11.9%, STS score 7.5±5.6%) (Table 1 and Table 2). TAVI was technically successful in all patients.
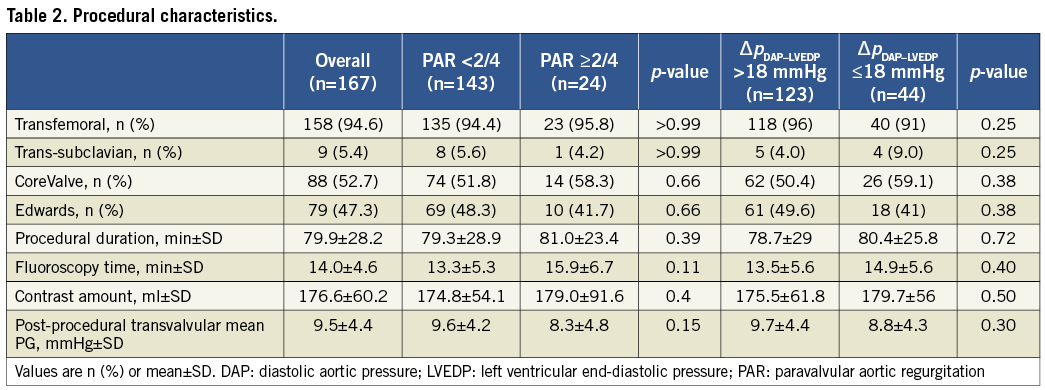
Immediately after valve implantation, PAR was judged by the operator as intolerable in 24 patients. Twenty-three patients underwent post-dilatation (MCV: n=3; ES: n=20) with an improvement in PAR grade to <2/4 in 13 (54%) of them. One patient with severe PAR due to low implantation of a MCV underwent post-deployment repositioning by snaring which resulted in PAR improvement to 1/4. After these corrective manoeuvres PAR was still present in 113 (67.7 %) and absent in 54 (32.3%) patients. Angiography revealed trivial or mild residual PAR in 89 (53.3%), moderate PAR in 21 (12.6%), and moderate-to-severe PAR in three (1.8%) patients; severe residual PAR did not occur.
Patients with relevant and non-relevant PAR did not differ in post-procedural systolic aortic pressure and LV systolic pressure (Table 3). However, DAP was significantly lower in patients with PAR ≥2/4 (p<0.001) and LVEDP higher (p=0.02), resulting in lower ∆PDAP-LVEDP gradient for patients with PAR ≥2/4 (p<0.001).
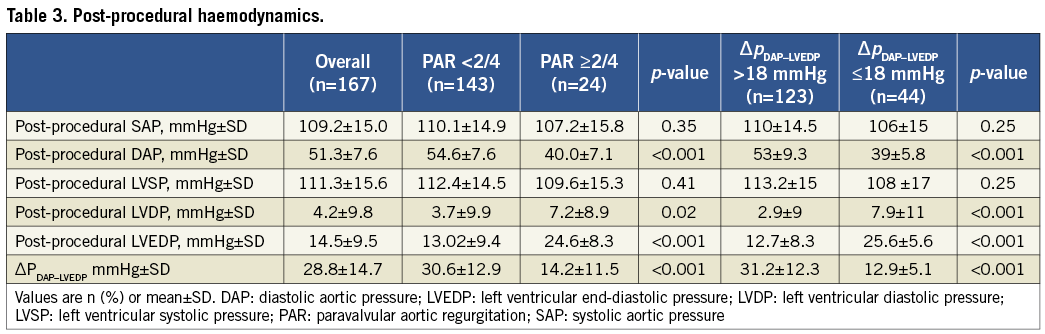
Residual PAR and ∆PDAP-LVEDP after TAVI were evaluated separately for each valve type. PAR was more often absent with the ES (41.8% vs. 23.9%, p=0.02), and mild PAR was seen more often with the MCV (60.2% vs. 45.6%, p=0.06). No and mild PAR (i.e., non-relevant PAR) were equally distributed between both valves (84.1% vs. 87.4%, p=0.6). Moderate-to-severe PAR occurred only with the MCV (3.4% vs. 0%) (Figure 2A). ∆PDAP-LVEDP of >39 mmHg was measured in 27.8% of patients with ES but only in 13.6% with MCV (p=0.03). ∆PDAP-LVEDP ≤18mm Hg was measured in 29.5% of patients with MCV and 22.8% with ES (p=0.3) (Figure 2B).
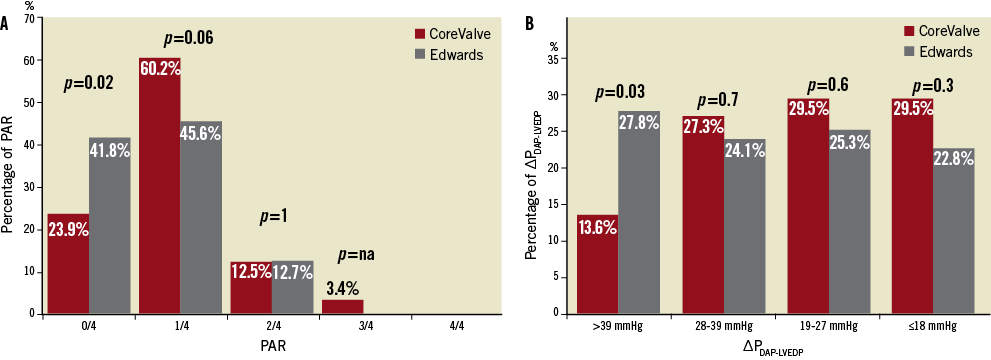
Figure 2. Comparison of the self-expandable and balloon-expandable prostheses with respect to PAR according to Sellers and ∆PDAP-LVEDP. The Figure shows a tendency for the MCV bioprosthesis to be associated with higher PAR (A) and less ∆PDAP-LVEDP (B) than the ES valve.
PAR AND MORTALITY AFTER TAVI
Mortality was 9% and 20% at 30 days and one year after TAVI, respectively. Cardiovascular mortality at 30 days and one year was increased in patients with moderate and moderate-to-severe PAR compared to those with no and mild PAR (46% vs. 4% and 73% vs. 7%, respectively, p<0.001). Patients with moderate and moderate-to-severe PAR had a lower rate of 30-day and one-year survival than patients with mild and absent PAR (log-rank <0.001) (Figure 3A). There was no difference in outcome between patients with no PAR compared to those with mild PAR.
When subdividing patients into quartiles according to ∆PDAP-LVEDP >39 mmHg (n=34), ∆PDAP-LVEDP=28-39 mmHg (n=43), ∆PDAP-LVEDP=19-27 mmHg (n=46), ∆PDAP-LVEDP≤18 mmHg (n=44), cardiovascular mortality at 30 days and one year was increased in patients with ∆PDAP-LVEDP≤18 mmHg compared to those with ∆PDAP-LVEDP>18 mmHg (21% vs. 6% and 32% vs. 13%, respectively, p=0.001). Patients with ∆PDAP-LVEDP≤18 mmHg had a lower rate of 30-day and one-year survival than patients with ∆PDAP-LVEDP>18 mmHg (log-rank=0.001) (Figure 3B). Using receiver operating characteristic curve analysis, a cut-off point of 18 mmHg proved to be an excellent predictor of mortality with a sensitivity of 80%, a specificity of 100% and an overall area under the curve (AUC) of 0.97 (Figure 4). Testing the value of the aortic regurgitation index which has been proposed by Sinning et al11 as a predictor of mortality using receiver operating characteristic curve analysis showed an AUC of 0.92. The comparison of the two AUCs delivered a p-value of 0.7. Therefore, no significant difference could be found between the new index of Sinning et al and ∆PDAP-LVEDP. Distribution of PAR grading as ordinal variables in relation to ∆PDAP-LVEDP using the chi-square test showed a significant correlation between these two parameters (p<0.001). The pressure difference sank in proportion to the rise of the percentage of PAR after Sellers: 17 of the 24 patients with moderate or moderate to severe PAR had ∆PDAP-LVEDP≤18 mmHg. The other seven patients were in the group with ∆PDAP-LVEDP=19-27 mmHg.
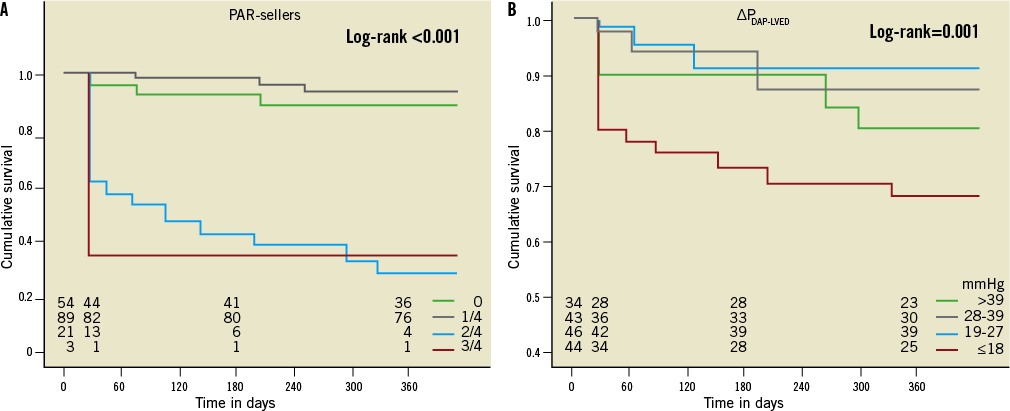
Figure 3. Cumulative survival by PAR grading using angiography (A) and ∆PDAP-LVEDP (B). Patients with moderate and moderate-to-severe PAR had a lower 30-day and one-year survival rate than patients with mild and no PAR (A). Patients with ∆PDAP-LVEDP ≤18 mmHg had a lower rate of 30-day and one-year survival than patients with ∆PDAP-LVEDP >18 mmHg (B).
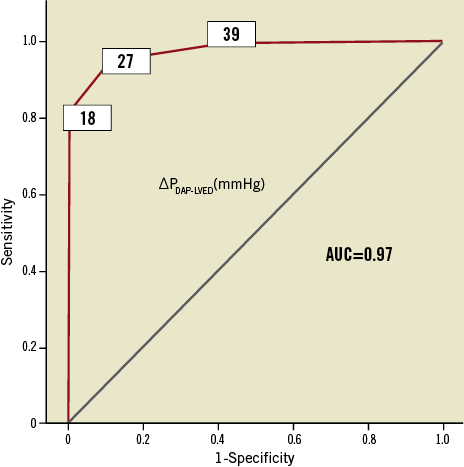
Figure 4. ROC analysis. ∆PDAP-LVEDP ≤18 mmHg is a good predictor of 30-day and one-year mortality.
Obviously, there was a relation between the Sellers and the ∆PDAP-LVEDP criteria to predict cardiovascular mortality which was increased in patients with at least moderate PAR when ∆PDAP-LVEDP was below 18 mmHg: 93% of the patients with at least moderate PAR who died of cardiovascular causes had ∆PDAP-LVEDP ≤18 mmHg.
Kaplan-Meier survival analysis in relation to the pre-existing aortic regurgitation, when classified as absent or mild vs. moderate or moderate-to-severe, was performed for patients with at least moderate PAR and ∆PDAP-LVEDP≤18 mmHg after TAVI. The impact of pre-existing aortic regurgitation on outcome between patients with at least moderate PAR after TAVI (log-rank=0.22) and patients with ∆PDAP-LVEDP≤18 mmHg (log-rank=0.81) was not significant.
PREDICTORS OF PAR AND CARDIOVASCULAR MORTALITY AFTER TAVI
Body height and aortic annulus diameter were associated with relevant PAR ≥2/4 and ∆PDAP-LVEDP≤18 mmHg using univariate analysis. These two (height and aortic annulus diameter) and six additional parameters (age, male gender, weight, aortic valve area before TAVI, left ventricular ejection fraction (LVEF), valve type) were included in the multivariate analysis but only height remained as an independent predictor of PAR (adjusted OR=1.107, 95% confidence interval: 1.01-1.191, p=0.02) and ∆PDAP-LVEDP≤18 mmHg (adjusted OR=1.080, 95% confidence interval: 1.013-1.152, p=0.019). Aortic valve area at baseline also predicted ∆PDAP-LVEDP ≤18 mmHg (adjusted OR=0.051, 95% confidence interval: 0.003-0.883, p=0.041) (Table 4).
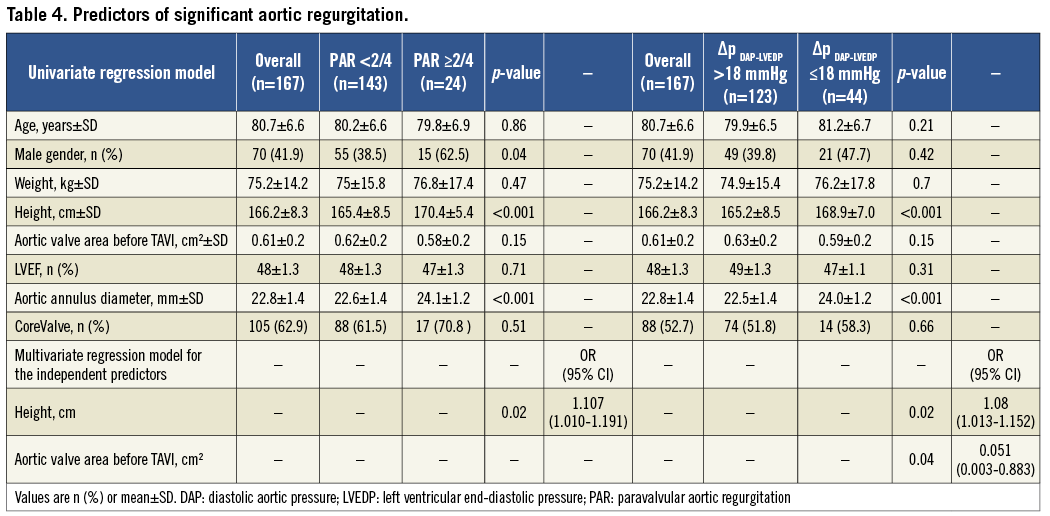
Only PAR ≥2/4 and ∆PDAP-LVEDP≤18 mmHg were associated with cardiovascular mortality at 30 days and one year using univariate analysis. The evaluation of other variables predicting cardiovascular mortality in the multivariate model was difficult since PAR ≥2/4 and ∆PDAP-LVEDP≤18 mmHg strongly separate deceased from living patients. Using multivariate analysis without these two parameters, only prior myocardial infarction predicted cardiovascular mortality at 30 days (adjusted OR=1.21, 95% confidence interval: 1.02-143.1, p=0.048) and no parameter predicted one-year mortality.
Discussion
In the present study we found PAR in the majority of patients after TAVI. Relevant PAR occurred in 14.4% of patients and was associated with increased cardiovascular mortality. Measurement of the pressure gradient ∆PDAP-LVEDP provided a quantitative assessment of PAR severity and was also associated with increased cardiovascular mortality: 93% of patients with PAR ≥2/4 by angiography who died of cardiovascular causes had ∆PDAP-LVEDP≤18 mmHg. Therefore, ∆PDAP-LVEDP≤18 mmHg is a novel predictor of cardiovascular mortality from PAR after TAVI.
PAR AFTER TAVI
AVR remains the first-line therapy for symptomatic aortic valve stenosis. TAVI is a viable alternative in selected high-risk patients with severe symptomatic aortic stenosis15. Obviously, the availability of several different prostheses and sizes allows the surgeon to select the most appropriate valve for an individual patient, whereas the narrow range of different sizes for both MCV and ES makes them sensitive to patient-prosthesis mismatch. The two currently CE-approved bioprostheses have similar forward flow haemodynamics to surgical bioprostheses, but result in more frequent PAR, approximately 50% for TAVI4,5 vs. approximately 30% for SAVR15-18. Some studies reported no association of PAR with mortality19,20, while others reported increased in-hospital and long-term mortality with moderate or severe PAR after TAVI5,9-11. However, more than mild aortic regurgitation after SAVR is also associated with haemodynamic burden and often requires reintervention1. The more frequent occurrence of PAR after TAVI than after SAVR obviously relates to the different procedures. During SAVR, the aortic valve is inspected, the diseased native tissue and calcified regions of the annulus excised, the annulus adjusted to the appropriate sewing ring, and an appropriate valve and its size selected21. With TAVI, the diseased, usually heavily calcified, native leaflets are crushed against the aortic annulus during expansion of the crimped stent-mounted prosthesis9, possibly preventing adequate stent-frame expansion and thus causing PAR22. Multimodality imaging is the gold standard to assess all required anatomical data in order to plan the procedures. However, during TAVI only indirect annulus sizing using echocardiography, CT or angiography is possible, and limited prosthesis sizes are currently available. Echocardiography probably underestimates annulus size23 so that potentially undersized valves are implanted. Also, the annulus is rather oval and not round24, and therefore sizing with two-dimensional imaging such as echocardiography can ‘‘cut’’ the oval plane at many angles, each resulting in a different estimate of annular diameter9,21. In patients with a large annulus, currently available prostheses might be undersized, resulting in annulus-device discrepancy18,25,26. Such a discrepancy might also explain the association between patient height and PAR in our study. In any event, careful imaging is necessary, and balloon sizing during valvuloplasty might help in more exact annular sizing27. In the present study, there was a tendency towards higher degrees of PAR and lower pressure gradients after MCV than ES implantation. The characteristics of the self-expandable nitinol frame and its potential deformation or incorrect site of prosthesis implantation (i.e., too low or too high) are potential explanations28,29. There was no impact of pre-existing aortic regurgitation on outcome of patients with at least moderate PAR after TAVI.
HAEMODYNAMIC GRADING OF PAR
We found ∆PDAP-LVEDP to be a novel and readily available predictor of cardiovascular mortality from PAR after TAVI. ∆PDAP-LVEDP is the driving pressure for myocardial perfusion, particularly the more ischaemia-vulnerable inner myocardial layers30, and ischaemia may be the link between PAR and mortality. As such, our ∆PDAP-LVEDP is similar to the somewhat more complex aortic regurgitation index proposed by Sinning et al11. ∆PDAP-LVEDP is simple to determine on-line and may therefore serve in decision-making regarding countermeasures such as repositioning using the “snare technique” or post-dilatation, especially in borderline cases. If patients do not respond to post-dilatation, valve-in-valve implantation must be considered19,31.
Limitations and strengths
Recent studies show an association between the amount and “asymmetry” of valvular and annular calcifications with the occurrence of PAR32. Although important, prediction of PAR was not the goal of our study. Due to the fact that important PAR predictors have not been included in the analysis, our index is only of limited value for PAR prediction. Nevertheless, the main purpose of the present analysis was to facilitate the on-table decision-making process as to whether residual PAR can be tolerated or requires further intervention. The four ∆PDAP-LVEDP quartiles in the present study are not equivalent to the four grades of the Sellers classification. In fact, there is some discrepancy between patients with PAR ≥2 (n=24) and patients with ∆PDAP-LVEDP≤18mmHg (n=44), and the 20 patients without relevant PAR who had ∆PDAP-LVEDP≤18mmHg. Conditions other than PAR that are frequently encountered in TAVI patients (e.g., reduced LVEF, concomitant mitral regurgitation, diastolic dysfunction) also contribute to increased LVEDP and consequently reduced ∆PDAP-LVEDP.
Conclusion
PAR after TAVI was observed in 67.7% of patients, and in 14.4% it was graded as moderate or moderate-to-severe. Angiographically moderate and moderate-to-severe PAR was associated with increased cardiovascular mortality. The transaortic pressure gradient ∆PDAP-LVEDP≤18 mmHg is a novel and easily available predictor of cardiovascular mortality in patients with PAR after TAVI.
Acknowledgements
We thank André Scherag, Institute for Medical Informatics, Biometry and Epidemiology, University Duisburg-Essen for statistical support.
Conflict of interest statement
M. Thielmann and P. Kahlert are clinical proctors for Edwards Lifesciences Inc. H. Eggebrecht is a clinical proctor for Medtronic Inc. The other authors have no conflicts of interest to declare.

