Abstract
Background: Mild paravalvular regurgitation (PVR) remains a frequent and underappreciated adverse event after transcatheter aortic valve implantation (TAVI) despite remarkable progress in device technology and implantation technique.
Aims: This study sought to investigate the impact of mild PVR after TAVI on five-year clinical outcomes.
Methods: In a prospective TAVI registry, PVR prior to discharge was retrospectively assessed in an echocardiographic core laboratory. Patients with ≥moderate PVR were excluded. Mild PVR was categorised into mild and mild-to-moderate PVR using a recently proposed unifying 5-class grading scheme.
Results: A total of 1,128 patients undergoing TAVI between 2007 and 2015 were enrolled. Of these, 560 patients had mild PVR, including 433 with mild (5-class) PVR and 127 with mild-to-moderate PVR. Patients with mild PVR were older (83 years vs 82 years, p=0.013) and had a higher surgical risk compared to patients with none/trace PVR (STS-PROM: 6.49±4.68 vs 5.41±3.48, p<0.001). At five years, patients with mild PVR had a higher risk of mortality than those with none/trace PVR (54.6% vs 43.8%; HRadjusted 1.26, 95% CI: 1.06-1.50). When applying the 5-class grading scheme, only mild-to-moderate PVR was associated with an increased risk of mortality at five years (mild PVR: HRadjusted 1.19, 95% CI: 0.99-1.43, mild-to-moderate PVR: HRadjusted 1.56, 95% CI: 1.20-2.02). The effect of mild PVR on five-year mortality was consistent across major subgroups.
Conclusions: Mild PVR was associated with an increased risk of mortality at five years after TAVI. The detrimental effect was primarily driven by mild-to-moderate PVR using the 5-class grading scheme. Clinical Trial Registration: https://www.clinicaltrials.gov. NCT01368250.
Introduction
The detrimental effect of moderate or greater paravalvular regurgitation after transcatheter aortic valve implantation (TAVI) is well established12345 and has instigated iterative refinement of valve designs. The introduction of sealing skirts, cuffs, and partial repositionability substantially mitigated the occurrence of moderate or greater paravalvular regurgitation (PVR) after TAVI. Indeed, recent trials in low-risk patient populations as well as real-world registries indicated comparable rates of higher (≥moderate) grade PVR after TAVR and surgical aortic valve replacement (SAVR)678. However, even in the recent trials using current-generation devices and implantation techniques, the rates of mild PVR have been reported to be as high as 30% to 36% after TAVI; substantially higher than those of surgical valves (3%)67. Mild PVR remains a significant concern related to TAVI compared with SAVR.
In contrast to moderate or greater PVR, the clinical importance of mild PVR remains a matter of debate12345. As both transfemoral TAVI and SAVR are recommended as first-line therapies for patients with severe aortic stenosis (AS) aged 65 years or older, according to recently issued guidelines of the American Heart Association and the American College of Cardiology9, the long-term effect of mild PVR is of paramount importance in guiding the Heart Team's decision, especially in lower-risk patient populations101112. Thus, in the present study, we aimed to investigate the clinical impact of mild PVR after TAVI on five-year outcomes in a prospective TAVI registry. Furthermore, we evaluated the validity of the discrimination of mild from mild-to-moderate PVR according to a recently proposed unifying 5-class grading scheme413, which is now included in the updated Valve Academic Research Consortium (VARC) definitions of clinical endpoints for TAVI trials14.
Methods
Study design and population
All patients undergoing TAVI at Bern University Hospital, Bern, Switzerland, are consecutively enrolled into a prospective institutional registry as part of the Swiss TAVI registry (NCT01368250)15. The registry was approved by the Bern cantonal ethics committee, and patients provided written informed consent for participation. The present analysis included all patients who underwent TAVI between August 2007 and December 2015. Patients were excluded if a non-CE marked device was used or if no transcatheter heart valve was implanted. For the purposes of the present analysis, patients who underwent TAVI for a degenerated surgical bioprosthesis or a previously implanted transcatheter bioprosthesis, those who underwent TAVI for pure native aortic valve regurgitation, those with periprocedural death before post-procedural echocardiography was performed, and those without adequate echocardiographic images for the assessment of PVR prior to discharge were excluded. Subsequently, patients with moderate or severe PVR were excluded from the primary analysis of the present study. This study conforms to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines16.
Assessment of PVR
Standardised transthoracic echocardiography was performed by a board-certified cardiologist and an echocardiography specialist with a Phillips iE33 machine (Philips Healthcare) at day 2 or 3 after TAVI and before hospital discharge, at the latest. The severity of PVR was independently re-evaluated by two investigators (T. Okuno and D. Tomii) in the Bern University cardiac imaging core laboratory by using a previously proposed 5-class grading scheme41314: “mild”, “mild-to-moderate”, “moderate”, “moderate-to-severe”, and “severe”. These five classes of grading were subsequently collapsed into a 3-class grading scheme (mild, moderate, severe): “mild” and “mild-to-moderate” in the 5-class grading scheme were collapsed into “mild”, and “moderate” and “moderate-to-severe” were collapsed into “moderate”. In accordance with the criteria for the grading scheme, a multiparametric and integrative approach was employed for the assessment of PVR. The evaluation relied more heavily on the width at the origin, the circumferential extent, the features, and the number of PVR jet(s), assessed visually in multiple views. The two investigators read together the first 50 cases to standardise the assessment before starting the core laboratory analyses.
Data collection and clinical endpoints
Baseline clinical, procedural, and follow-up data were prospectively entered into a dedicated web-based database. Preprocedural computed tomography (CT) examinations were independently re-evaluated by dedicated imaging specialists and the measurements were integrated into the database. Device landing zone calcium volume (mm3) was quantified as previously validated1718. Clinical follow-up data were obtained by the use of standardised interviews, documentation from referring physicians, and hospital discharge summaries. In the Swiss TAVI registry, regular follow-up is standardised at 30 days, 1 year, and 5 years. A clinical events committee independently adjudicated all adverse events on the basis of the VARC criteria14. An independent Clinical Trials Unit is responsible for central data monitoring to verify completeness and accuracy of data and independent statistical analysis.
The outcomes of interest for the present study included all-cause mortality, cardiovascular mortality, any stroke (disabling or non-disabling), major or life-threatening bleeding, structural valve deterioration, and unplanned repeat aortic valve intervention at 1 and 5 years. Functional status was assessed by New York Heart Association (NYHA) Class at 1 and 5 years. Unplanned repeat aortic valve intervention was defined as a composite endpoint including valve-in-valve procedure, balloon valvuloplasty, surgical revision, or paravalvular leak closure.
Statistical analysis
Categorical variables are represented as frequencies and percentages and the differences between groups were evaluated with the chi-square test or Fisher’s exact test. Rate ratios (RR) with 95% confidence intervals (CI) from Poisson regressions were provided where appropriate. Continuous measures are presented as mean values±standard deviation (SD) and compared between groups using a t-test. Time-to-event curves are depicted using the Kaplan-Meier method. Univariable and multivariable Cox proportional hazards models were used to calculate hazard ratios (HR) and 95% CIs for clinical outcomes. Baseline variables entered into the multivariable model for adjustment were predefined based on the presumed association with clinical outcomes of interest: age, gender, body mass index, Society of Thoracic Surgeons Predicted Risk of Mortality (STS-PROM), and NYHA Class III or IV. As a sensitivity analysis, a post hoc multivariable adjustment by adding baseline variables that were significantly different between the groups, and device landing zone calcium as assessed by CT to the predefined model, was performed in patients with assessable CT images (n=844). In all time-to-event analyses, data for a patient were censored at the time of the first event that occurred in that patient. Throughout the study, statistical significance was defined at a two-sided p-value of <0.05. All statistical analyses were performed with the use of Stata 15.1 (StataCorp).
Results
Studied population and baseline characteristics
Among 1,321 consecutive patients who underwent TAVI between August 2007 and December 2015, 1,202 patients had adequate raw echocardiographic clips after TAVI, prior to discharge, for the assessment of PVR. Of these, 74 patients (6.2%) had moderate or greater PVR and were excluded from the primary analysis. As a result, 1,128 subjects, including 568 patients (47.3%) with none/trace PVR and 560 patients (46.6%) with mild PVR (3-class), comprised the study population and were analysed. In the 5-class grading scheme, 560 patients with mild PVR (3-class) were categorised into 433 patients (36.0%) with mild PVR (5-class) and 127 patients (10.6%) with mild-to-moderate PVR (5-class) (Figure 1).
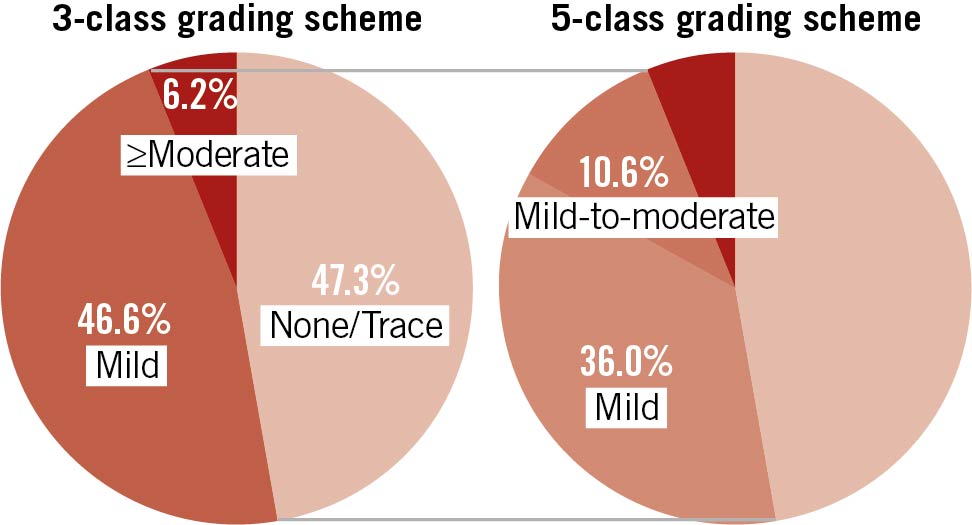
Figure 1. Incidence of paravalvular regurgitation (PVR) after TAVI according to 3-class and 5-class grading scheme. Pie chart illustrating the proportions of PVR according to 3-class and 5-class grading schemes. In the unifying 5-class grading scheme, mild PVR is subdivided into mild and mild-to-moderate PVR.
Baseline clinical, echocardiographic, and procedural characteristics of the study population are summarised in Table 1. Patients with mild PVR prior to discharge were older (82.7±6.0 years vs 81.8±5.6 years, p=0.013), had a lower body mass index (25.9±5.1 kg/cm2 vs 27.1±5.2 kg/cm2, p<0.001) and a higher surgical risk (STS-PROM 6.49±4.68 vs 5.41±3.48, p<0.001) than patients with none/trace PVR. On baseline echocardiography, patients with mild PVR had a smaller aortic valve area (0.65±0.22 cm2 vs 0.68±0.25 cm2, p=0.023), and a lower left ventricular ejection fraction (LVEF) (53.1±15.0% vs 55.9±14.2%, p=0.002) than patients with none/trace PVR. On CT, there was no difference in the prevalence of bicuspid anatomy (5.6% vs 4.7%, p=0.647), whereas the volume of device landing zone calcium was significantly larger in patients with mild PVR than in those with none/trace PVR (384.4±374.8 mm3 vs 297.7±315.6 mm3, p<0.001). With regard to procedural characteristics, self-expanding devices were more frequently used (52.7% vs 34.3%, p<0.001), and predilation and post-dilation were more frequently performed in patients with mild PVR than in patients with none/trace PVR.
Clinical outcomes according to the 3-class grading scheme
Clinical follow-up was complete in 1,104 patients (97.9%) at 1 year and 1,085 patients (96.2%) at 5 years. Clinical outcomes at 1 and 5 years according to the presence or absence of mild PVR prior to discharge are shown in Table 2. At 5 years, patients with mild PVR prior to discharge had a higher risk of all-cause mortality than patients with none/trace PVR (54.6% vs 43.8%, HRadjusted 1.27, 95% CI: 1.07-1.51; p=0.007) (Central illustration). At 1 year, all-cause mortality occurred in 14.7% of patients with mild PVR and in 11.1% of patients with none/trace PVR (HRadjusted 1.19, 95% CI: 0.84-1.67; p=0.329). Major or life-threatening bleeding occurred more frequently in patients with mild PVR than in those with none/trace PVR both at 1 and 5 years (HRadjusted 1.49, 95% CI: 1.16-1.90; p=0.001, and HRadjusted 1.51, 95% CI: 1.20-1.90; p<0.001, respectively). There were no significant differences in the rates of stroke, structural valve deterioration, and unplanned repeat aortic valve intervention at 1 and 5 years. In a sensitivity analysis, although the effect of mild PVR on five-year all-cause mortality was marginally significant (HRadjusted 1.22, 95% CI: 0.99-1.50; p=0.060), the results were largely consistent (Supplementary Table 1). The proportion of patients with NYHA Class III or IV heart failure symptoms was comparable between groups at 1 and 5 years. In subgroup analyses according to clinical and echocardiographic parameters, there was a consistent association between mild PVR and higher five-year all-cause mortality (Figure 2). A Kaplan-Meier curve for all-cause mortality according to the severity of PVR, including moderate PVR, is shown in Supplementary Figure 1.
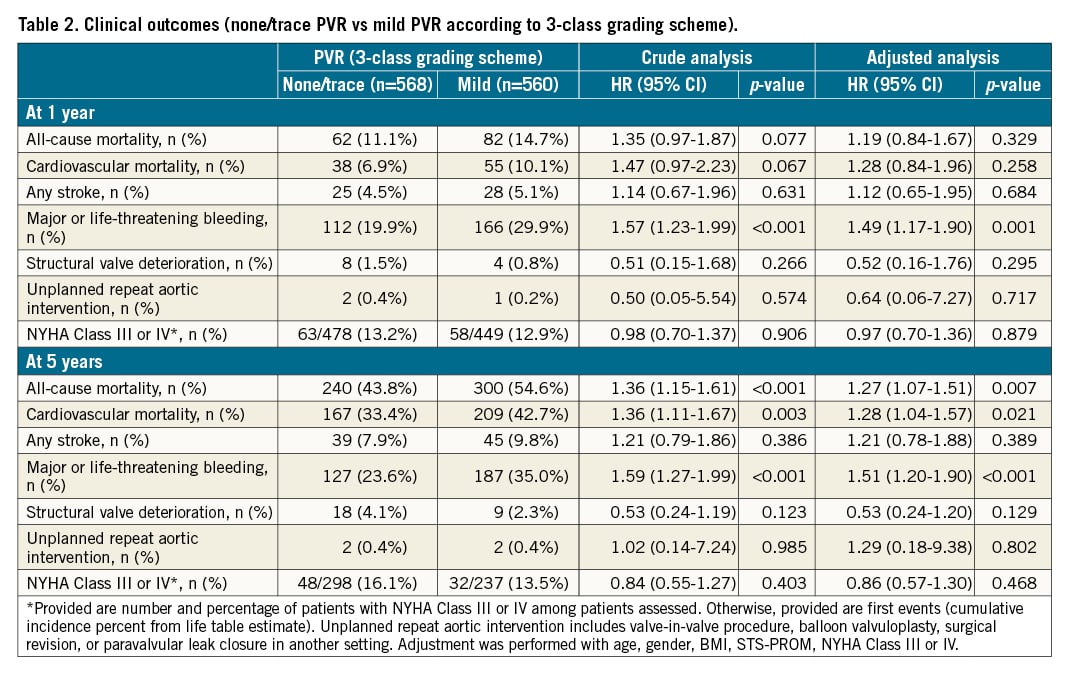
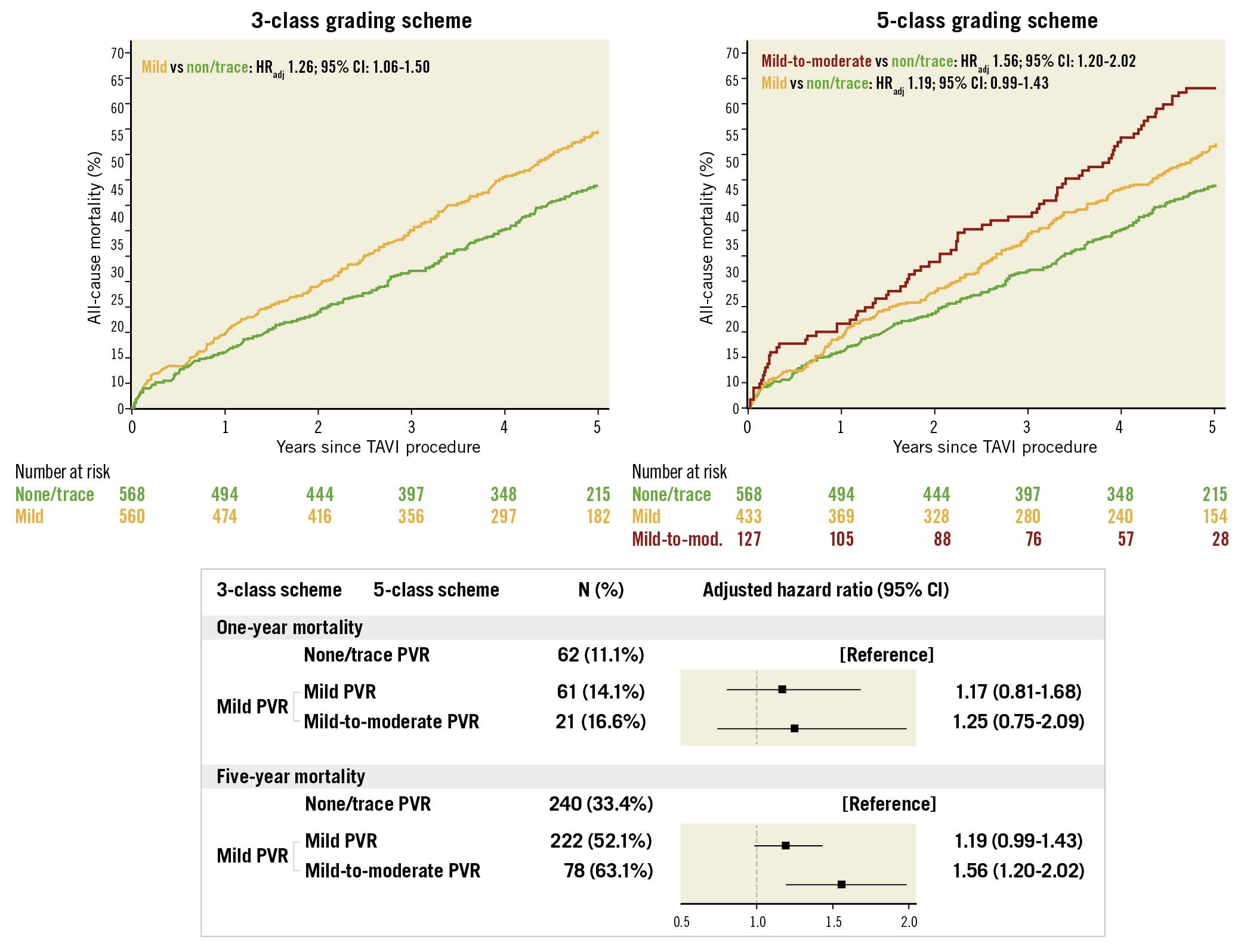
Central illustration. Impact of mild PVR on all-cause mortality according to the 3-class and 5-class grading schemes. Kaplan-Meier curves for all-cause mortality in patients with none/trace PVR (green) and mild PVR (yellow) according to the 3-class grading scheme and in patients with none/trace PVR (green), mild PVR (yellow), and mild-to-moderate PVR (red) according to the 5-class grading scheme. Forest plots summarise the impact of mild PVR on mortality. Adjustment was performed with age, gender, BMI, STS-PROM, NYHA Class III or IV.
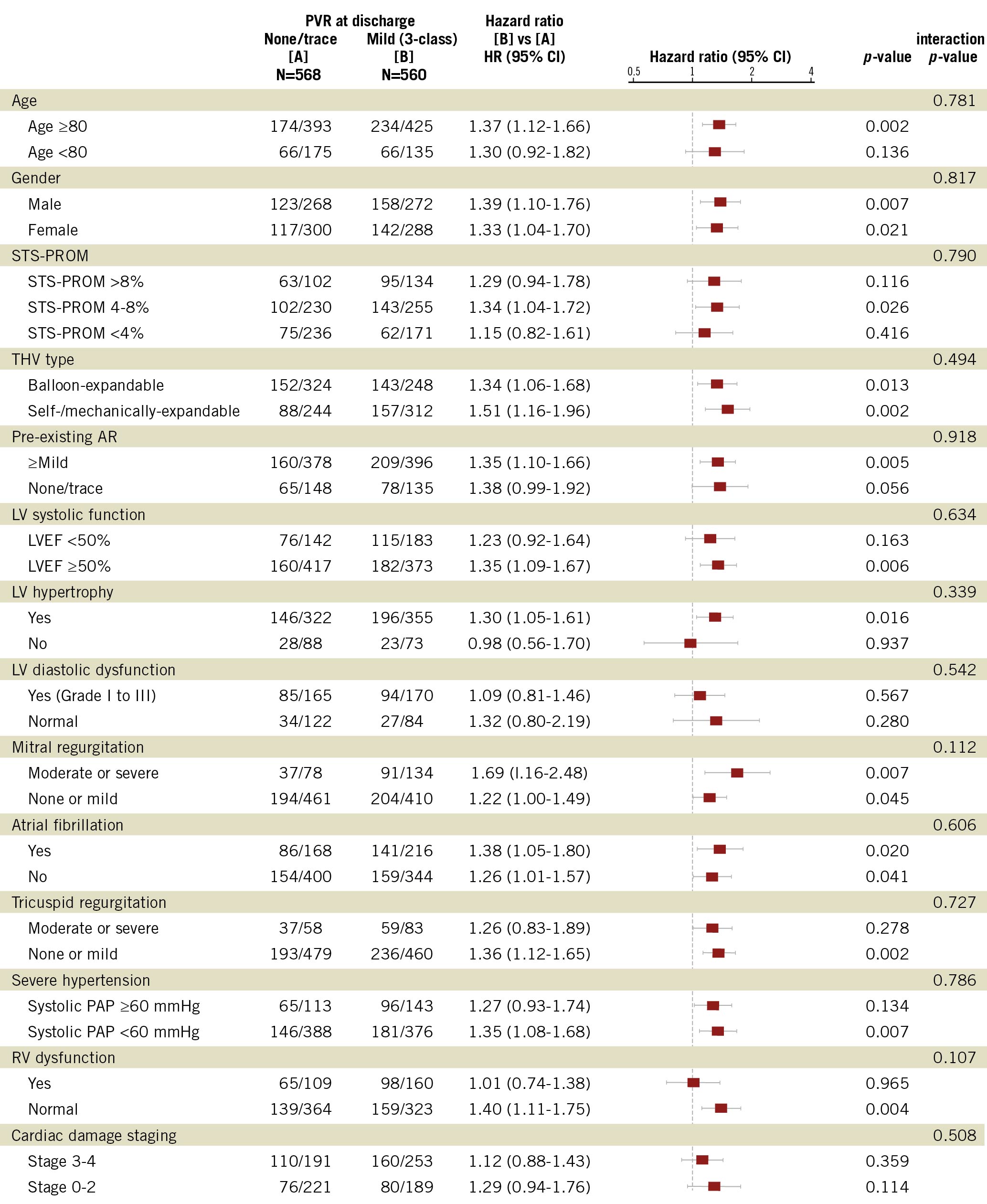
Figure 2. Subgroup analyses of the effect of mild PVR on five-year mortality. LV hypertrophy, LV diastolic dysfunction, RV dysfunction were defined in accordance with echocardiographic guidelines2829. The definitions of cardiac damage staging have been described previously30. AR: aortic regurgitation; LV: left ventricular; LVEF: left ventricular ejection fraction; PAP: pulmonary artery pressure; RV: right ventricular dysfunction; STS-PROM: Society of Thoracic Surgeons Predicted Risk of Mortality; THV: transcatheter heart valve
Clinical outcomes according to the 5-class grading scheme
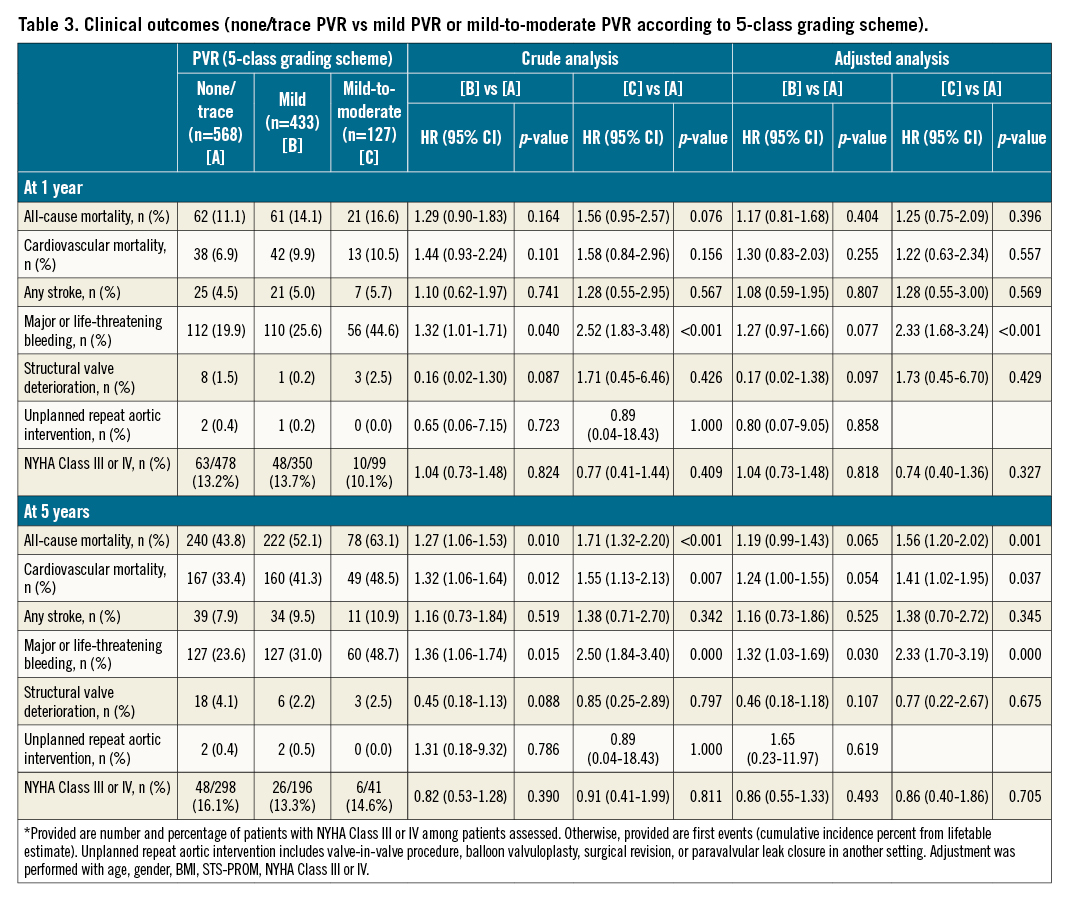
Clinical outcomes at 1 and 5 years stratified by none/trace, mild, and mild-to-moderate PVR using a 5-class grading scheme are shown in Table 3. At 5 years, all-cause mortality occurred in 63.1% of patients with mild-to-moderate PVR, in 52.1% of patients with mild PVR (5-class), and in 43.8% of patients with none/trace PVR (Central illustration). After multivariable adjustment, mild-to-moderate PVR conferred an increased risk of five-year all-cause mortality compared to none/trace PVR (HRadjusted 1.56, 95% CI: 1.20-2.02; p=0.001), while the risk was comparable between mild PVR (5-class) and none/trace PVR (HRadjusted 1.19, 95% CI: 0.99-1.43; p=0.065). In the one-year analysis, both patients with mild-to-moderate PVR and mild PVR (5-class) had a comparable risk of all-cause mortality as patients with none/trace PVR (HRadjusted 1.25, 95% CI: 0.75-2.09; p=0.396, and HRadjusted 1.17, 95% CI: 0.81-1.68; p=0.404, respectively) (Central illustration). At 5 years, major or life-threatening bleeding occurred more frequently in patients with mild-to-moderate PVR (48.7%; HRadjusted 2.33, 95% CI: 1.70-3.19; p<0.001) and in patients with mild PVR (5-class) (31.0%; HRadjusted 1.32, 95% CI: 1.03-1.69; p=0.030) compared to patients with none/trace PVR (23.6%). At 1 year, the risk was significantly higher in patients with mild-to-moderate PVR (44.6%; HRadjusted 2.33, 95% CI: 1.68-3.24; p<0.001), while the risk was comparable in patients with mild PVR (5-class) (25.6%; HRadjusted 1.27, 95% CI: 0.97-1.66; p=0.077) and patients with none/trace PVR (19.9%). There were no significant differences in the rates of stroke, structural valve deterioration, and unplanned repeat aortic valve intervention between patients with mild-to-moderate or mild (5-class) PVR and those with none/trace PVR at 1 and 5 years, respectively. The rates of NYHA Class III or IV heart failure symptoms were comparable between groups at 1 and 5 years. The results were consistent in a sensitivity analysis (Supplementary Table 2).
Discussion
The principal findings of this study are as follows. 1) Mild PVR was associated with an increased risk of all-cause mortality at five years after TAVI. 2) The effect of mild PVR on mortality was consistent, irrespective of patient baseline clinical or echocardiographic characteristics. 3) One fourth of mild PVR was classified as mild-to-moderate PVR in the 5-class grading scheme. 4) Mild-to-moderate PVR conferred a robust 1.6-fold increased risk of five-year mortality compared to none/trace PVR, while the effect of mild PVR (5-class) on five-year mortality was attenuated and with only borderline significance.
Moderate or greater PVR has consistently been associated with an increased risk of mortality after TAVI1234. Conversely, the effect of mild PVR has been controversial and may only be substantiated during extended follow-up. Due to the expansion of TAVI to patients with a longer life expectancy9, the potential impact of mild PVR on longer-term outcomes may become increasingly important. Recently, the impact of mild PVR on five-year outcome has been reported from some of the randomised strategy trials. In the high-risk trials, the presence of mild PVR was associated with higher mortality at five years1011. Conversely, in the PARTNER-2 trial, mild PVR was not associated with an increased risk of mortality at five years; however, there was a progressive divergence of time-to-event curves for all-cause mortality among patients with mild PVR and those with none/trace PVR, suggesting a possible delayed effect of mild PVR on clinical outcomes12.
Several factors have been proposed to explain the conflicting results of the impact of mild PVR on clinical outcomes. First, the accurate assessment of PVR requires several echocardiographic views which are frequently challenging to interpret due to artefacts from prosthetic valve material. In addition, interpretation largely relies on qualitative and semiquantitative parameters. Thus, the assessment may differ across centres or studies19. Second, “mild PVR” according to current guideline recommendations includes a broad range and may fail to discriminate clinical impact at a more granular level1320. Lastly, potential differences in tolerability against PVR across TAVI populations have been suggested. A protective effect of pre-existing aortic regurgitation or left ventricular (LV) dilatation have been suggested in previous studies321222324. Recently, a new grading scheme has been proposed to improve the assessment of severity and provide uniformity in the assessment of PVR41314. In an analysis of the PARTNER-2 registry using the 5-class grading scheme, only moderate or greater PVR was associated with an increased risk of death; clinical outcome was, however, assessed at one year and the number of individuals with mild-to-moderate PVR was modest (n=47)15. Later effects of low grade PVR were not evaluated.
In the present study, the effect of mild PVR (3-class) on mortality was not statistically significant at one year, consistent with previous reports1234. The detrimental effect of mild PVR accrued over time and emerged during extended follow-up. At five years, mild PVR (3-class) conferred a 1.3-fold increased risk of death after adjustment for differences in baseline characteristics. Intriguingly, when applying the 5-class grading scheme, mild-to-moderate PVR was significantly associated with an increased risk of mortality at five years, whereas the effect of mild PVR (5-class) on mortality risk was borderline significant only. Consistent with the PARTNER-2 SAPIEN 3 registry4, mild-to-moderate PVR did not affect mortality at one year. Furthermore, the effect of mild PVR on mortality was consistent across subgroups according to pre-existing aortic regurgitation and LV function.
In a continued access registry of the PARTNER-1 trial, moderate or severe PVR emerged as the strongest predictor of late bleeding events25. The loss of high-molecular weight von Willebrand factor due to high shear stress and flow turbulence caused by PVR has been proposed as an underlying mechanism26. In the present study, both mild and mild-to-moderate PVR, according to the 5-class grading scheme, were associated with a 1.3-fold and 2.3-fold increased risk of major or life-threatening bleeding at five years, respectively, suggesting that the effect of mild PVR on late bleeding events may have been underappreciated.
Our findings have important clinical implications for the management of severe AS, particularly in younger and low-risk populations. As even mild PVR according to the 3-class grading scheme has a significant impact on mortality at five years, every effort should be made to avoid mild PVR in younger and low-risk patients. Given that the rate of mild PVR is remarkably lower after SAVR than TAVI, even in recent trials using current-generation devices, SAVR may be favoured in younger patients who are considered at risk of mild or greater PVR18. Furthermore, the 5-class grading scheme may be particularly useful in this setting. If mild PVR is deemed to be mild-to-moderate PVR according to the proposed scheme, additional interventions, including post-dilation, valve-in-valve procedure, paravalvular leak closure, and even surgical revision, may be considered to improve longer-term outcomes.
Study limitations
Several limitations should be acknowledged when interpreting the results of the present study. First, the study was based on a cohort including patients who underwent TAVI between 2007 and 2015. Thus, the study included early-generation devices which are no longer in clinical use, and the observed rates of PVR may not be representative for contemporary devices. Nevertheless, the effect of individual grades on late clinical events is not a function of valve type. Moreover, the robustness of our findings is underscored by the prospective data collection, completeness of follow-up at five years of more than 96%, and independent event adjudication. Second, follow-up echocardiography was incompletely recorded in this registry. Thus, the change in PVR severity during follow-up could not be evaluated. Some studies reported regression of PVR at follow-up427, which may affect the long-term clinical impact of PVR. This should be further evaluated in future studies. Furthermore, the occurrence of structural valve deterioration may be underreported. Finally, the studied population in the present analysis was limited to elderly patients (mean age >80 years), hence the results may not be generalisable to younger patients. However, there was no significant interaction between age >80 years and the effect of mild PVR on mortality.
Conclusions
Mild PVR prior to discharge according to the 3-class grading scheme was associated with an increased risk of mortality at five years after TAVI. A recently proposed 5-class grading scheme accurately discriminated the prognostic implications of mild PVR. When applying the 5-class grading scheme, the upper range of mild PVR (mild-to-moderate PVR) was significantly associated with a 1.6-fold increased risk of mortality at five years, while the lower range of mild PVR conferred a borderline significant 1.2-fold increased risk. The long-term consequences of mild PVR have important repercussions on decision making between TAVI and SAVR, particularly in younger patients.
Impact on clinical practice
Mild PVR has a significant impact on five-year mortality in patients undergoing TAVI. The risk of mild PVR should be taken into account for decision making between TAVI and SAVR, particularly in younger patients with low to intermediate surgical risk. Furthermore, the 5-class grading scheme may be beneficial in patients with mild PVR. If mild PVR is deemed to be mild-to-moderate PVR according to the scheme, additional interventions, including post-dilation, valve-in-valve procedure, paravalvular leak closure, and even surgical revision, may be considered to improve longer-term outcomes.
Conflict of interest statement
S. Windecker reports research and educational grants to the institution from Abbott, Amgen, AstraZeneca, BMS, Bayer, Biotronik, Boston Scientific, Cardinal Health, Cardiovalve, CSL Behring, Daiichi Sankyo, Edwards Lifesciences, Guerbet, Infraredx, Johnson & Johnson, Medicure, Medtronic, Novartis, Polares, OrPha Suisse, Pfizer, Regeneron, Sanofi-Aventis, Sinomed, Terumo, and V-Wave. S. Windecker serves as an unpaid advisory board member and/or unpaid member of the steering/executive group of trials funded by Abbott, Abiomed, Amgen, AstraZeneca, BMS, Boston Scientific, Biotronik, Cardiovalve, Edwards Lifesciences, MedAlliance, Medtronic, Novartis, Polares, Sinomed, V-Wave and Xeltis, but has not received personal payments from pharmaceutical companies or device manufacturers. He is also a member of the steering/executive committee group of several investigator-initiated trials that receive funding from industry without impact on his personal remuneration. S. Windecker is an unpaid member of the Pfizer Research Award selection committee in Switzerland and of the Women as One Awards Committee. He is a member of the Clinical Study Group of the Deutsches Zentrum für Herz Kreislauf-Forschung and of the Advisory Board of the Australian Victorian Heart Institute. He is chairperson of the ESC Congress Program Committee and Deputy Editor of JACC Cardiovascular Interventions. T. Pilgrim reports research grants to the institution from Edwards Lifesciences, Boston Scientific and Biotronik, personal fees from Biotronik and Boston Scientific, proctoring for Medtronic (Evolut), travel support from Medira, and other from HighLife SAS. D. Reineke reports travel and congress expenses from Abbott, Edwards Lifesciences, and Medtronic. D. Reineke is a proctor for Abbott. S. Stortecky reports research grants to the institution from Edwards Lifesciences and Medtronic, and personal fees from Boston Scientific, Teleflex, and BTG. F. Praz reports travel expenses from Abbott, Edwards Lifesciences, and Polares Medical. T. Okuno reports speaker fees from Abbott. The other authors have no conflicts of interest to declare relevant to the contents of this article.
Supplementary data
To read the full content of this article, please download the PDF.

