Abstract
Background: Moderate or worse paravalvular regurgitation (PVR) post transcatheter aortic valve replacement (TAVR) is associated with increased mortality. The mechanisms by which this occurs are not fully understood.
Aims: The aim of this study was to determine the mechanism by which PVR leads to worse outcomes.
Methods: A total of 1,974 intermediate-risk patients who received TAVR in the PARTNER 2 trial and registries were grouped by PVR severity. Clinical and echocardiographic outcomes were compared.
Results: Overall 1,176 (60%) patients had none/trace, 680 (34%) had mild, and 118 (6%) had ≥moderate PVR. At two years, ≥moderate PVR patients had increased risks of all-cause (HR 2.33 [1.41-3.85], p-value=0.001) and cardiovascular death (HR 3.30 [1.74-6.28], p-value <0.001), rehospitalisation (HR 2.68 [1.57-4.58], p-value <0.001), and reintervention (HR 14.72 [3.13-69.32], p-value <0.001). Moderate or worse PVR was associated with larger increases in left ventricular (LV) end-diastolic and systolic dimensions and volumes, LV mass indices, and reductions in LV ejection fractions (LVEFs) from 30 days to two years. Mild PVR was not associated with worse outcomes. Adjusting for LV dimensions and LVEF from the one-year echocardiogram, patients with ≥moderate PVR still had an increased risk of all-cause death or rehospitalisation at two years (HR 2.84 [1.25-5.78], p-value=0.009).
Conclusions: Moderate or worse PVR, but not mild PVR, is associated with an increased risk of all-cause and cardiovascular death, rehospitalisation, and reintervention at two years. Moderate or worse PVR is also associated with adverse LV remodelling, which partially mediates how ≥moderate PVR leads to worse outcomes. These results provide dual insights on the deleterious impact of ≥moderate PVR and the contributing mechanisms of poor clinical outcomes.
Introduction
Paravalvular regurgitation (PVR) after transcatheter aortic valve replacement (TAVR) is an important complication associated with higher mortality and rehospitalisation rates123. Rates of ≥moderate PVR have decreased from the first-generation heart valves (5-38%) to the more recent prostheses with a prevalence of <5%24567. Despite this lower prevalence, ≥moderate PVR in patients with SAPIEN 3 (Edwards Lifesciences) valves was still associated with a more than twofold increase in mortality2.
Despite numerous studies consistently showing ≥moderate PVR to be associated with increased mortality, little is known about the mechanisms by which PVR leads to worse outcomes. Blunted left ventricular (LV) mass regression and increased LV dimensions post-TAVR, and pre-existing aortic regurgitation (AR) have all been found to be associated with either ≥moderate PVR or mortality among patients with ≥moderate PVR. However, no study has yet shown what is the mediator between ≥moderate PVR and outcomes189.
In this study, we pool together intermediate-risk patients from the PARTNER 2A trial and the S3i registry who received TAVR in order to investigate the mechanism by which PVR leads to worse clinical outcomes by looking at associated changes in echocardiographic parameters. Understanding the mechanisms by which PVR leads to worse clinical outcomes may help tailor therapies towards patients who develop ≥moderate PVR post-TAVR or may help identify patient subgroups who are at higher risk of developing poor outcomes with PVR.
Methods
Study design and population
This study consisted of all intermediate-risk patients who received TAVR from the PARTNER 2A trial and the PARTNER S3 intermediate risk (S3i) registry, the details of which have been published previously710. Briefly, in the PARTNER 2A cohort, intermediate-risk patients (predicted risk of 30-day mortality ≥4%, based on either the Society of Thoracic Surgeons [STS] score or clinical assessment by a multidisciplinary Heart Team) who were randomised to TAVR received the SAPIEN XT valve (Edwards Lifesciences). In the S3i registry, intermediate-risk patients (Society of Thoracic Surgeons [STS] score 4-8%) underwent TAVR with the SAPIEN 3 valve. In all cohorts, patients had severe symptomatic aortic stenosis (AS). The protocols were approved by the institutional review boards of each participating site, and all patients provided written informed consent.
Echocardiographic assessment of PVR
All baseline and follow-up transthoracic echocardiograms (TTEs) were read by a core laboratory. PVR severity was determined based on the TTE performed at 30 days post-TAVR. Patients with missing 30-day TTE data were imputed using discharge TTE results. PVR severity was graded using a multiparameter integrative approach, as previously described11. Based on prior literature, patients were divided into those with ≤mild PVR and those with ≥moderate PVR. For sensitivity analyses, ≤mild PVR patients were further divided into those with mild and none/trace PVR.
Endpoints
Clinical and echocardiographic outcomes up to two years post-TAVR were compared between groups. Clinical events were adjudicated by a clinical events committee. Clinical outcomes included all-cause mortality, cardiovascular (CV) mortality, rehospitalisation, reintervention, and stroke. Echocardiographic outcomes were assessed by the mean difference between the 30-day (or discharge) TTE and the one- and two-year TTE.
Statistical analysis
Continuous data were compared by the Student’s t-test and categorical data by the chi-squared test. Time-to-event variables were summarised by the number of events and Kaplan-Meier event rates and compared using the log-rank test. Clinical events occurring prior to collection of the 30-day/discharge TTE were censored to correct for immortal time bias.
Primary analysis of clinical outcomes was performed using study-stratified multivariable Cox proportional hazards regression models. Since there was only one type of transcatheter heart valve used within each study (i.e., SAPIEN XT or SAPEN 3), stratifying the models by study also stratifies the models by type of valve. Echocardiographic changes between the 30-day (or discharge) TTE and the one- and two-year TTE were analysed using baseline corrected analysis of covariance models and reported using least-square means and 95% confidence intervals (CIs). Confounding factors that were included in all multivariable models are listed in Supplementary Appendix 1. Propensity score-adjusted models were performed as a sensitivity analysis. A propensity score was derived for each patient by fitting a logistic regression model to predict the conditional probability of ≥moderate versus ≤mild PVR, adjusting for all covariates included in the multivariable models.
To examine whether PVR was associated with long-term mortality, a multivariable Cox proportional hazards landmark model was fitted for patients who remained alive at one year, which included PVR and known confounder variables as predictors of death or heart failure hospitalisation between 365 and 730 days. To examine whether PVR-related changes in LV morphology at one year were part of the mechanism by which PVR is associated with long-term risk of death or rehospitalisation, another multivariable Cox proportional hazards landmark model was fitted. This model was identical to the former model, with the exception that variables that were of interest as possible mechanisms—indices of changes in LV dimensions/function between baseline and 365 days—were added as covariates. Specifically, these variables were changes in left ventricular ejection fraction (LVEF), left ventricular (LV) end-diastolic diameter, and LV end-systolic diameter between baseline and 365 days post-TAVR. This allowed us to examine whether PVR remained an independent predictor of worse prognosis after adjustment for changes in LV dimensions and function. All statistical analyses were performed using SAS version 9.4 (SAS Institute).
Results
Patient population
Of 1,974 patients included in this study, 947 were from the PARTNER 2A cohort and 1,027 were from the S3i registry. A total of 1,176 (60%) patients had none/trace PVR, 680 (34%) had mild PVR, and 118 (6%) had ≥moderate PVR. Of the ≥moderate PVR patients, 9 had severe PVR (one of whom underwent reintervention). When patients were grouped into those with ≤mild PVR and those with ≥moderate PVR, ≥moderate PVR patients were older, had a lower body mass index (BMI), higher STS scores, and a lower prevalence of diabetes (Table 1). More ≥moderate PVR patients had the SAPIEN XT valve implanted, while more ≤mild PVR patients had the SAPIEN 3 valve implanted.
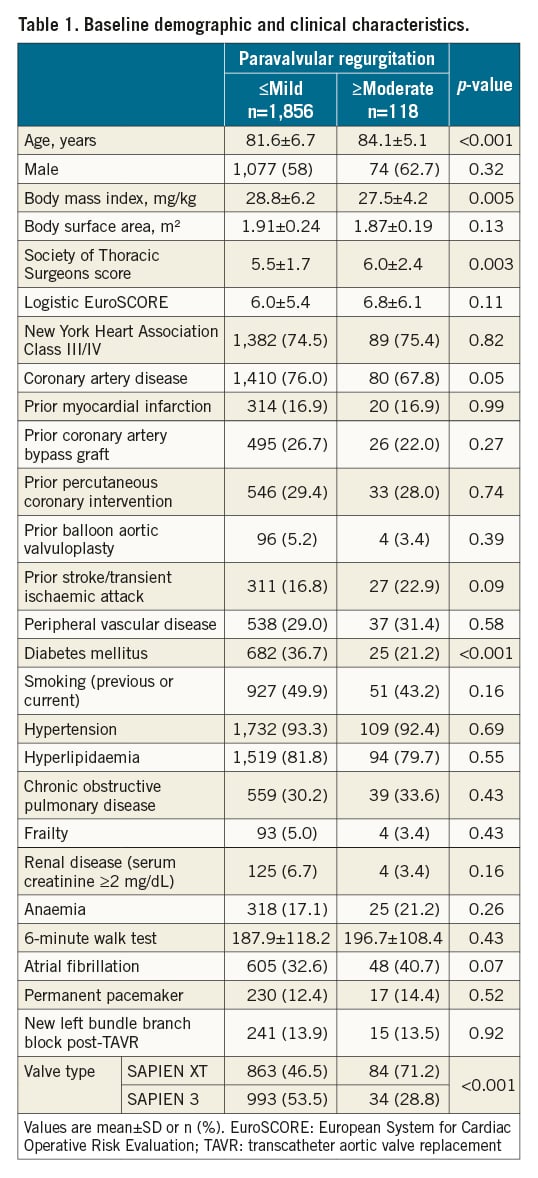
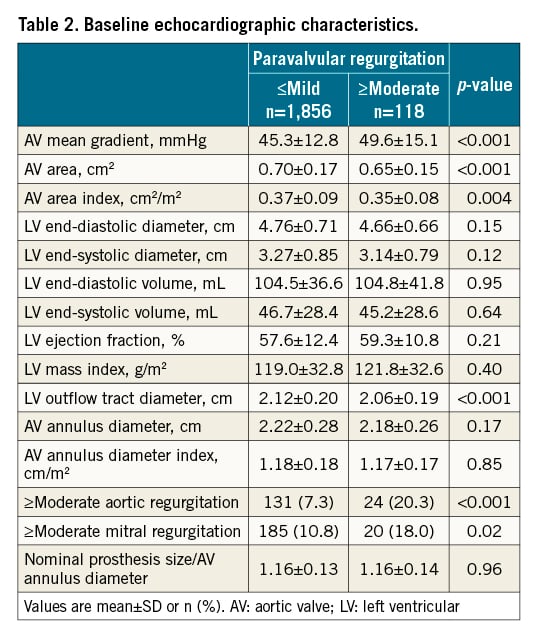
Echocardiographic data at baseline are shown in Table 2. Patients with ≥moderate PVR had higher aortic valve (AV) mean gradients, AV areas and area indices, and smaller LV outflow tract diameters. They also had a significantly higher prevalence of ≥moderate aortic and mitral regurgitation. Otherwise, both groups had similar LV dimensions, LVEFs, and AV annulus diameter indices. More ≤mild PVR patients had a 29 mm valve implanted, less post-dilation, and a lower incidence of multiple valves implanted (Supplementary Table 1).
PVR severity and echocardiographic outcomes
Moderate or worse PVR patients had significant echocardiographic changes between 30 days and one year post-TAVR, resulting in larger LV dimensions and volumes, and lower LVEFs compared with ≤mild PVR patients (Supplementary Table 2). Moderate or worse PVR patients also had an increase in LV mass index while ≤mild PVR patients experienced LV mass index regression. These changes continued to progress up to two years, as seen in both unadjusted (Supplementary Table 3) and adjusted analyses (Central illustration). At two years, ≥moderate PVR patients had significantly larger LV dimensions and volumes while they were similar or somewhat smaller in ≤mild PVR patients. Moderate or worse PVR patients experienced a greater decrease in LVEF between 30 days and two years than ≤mild PVR patients. By two years, ≥moderate PVR patients experienced some LV mass index regression, though significantly less than ≤mild PVR patients.
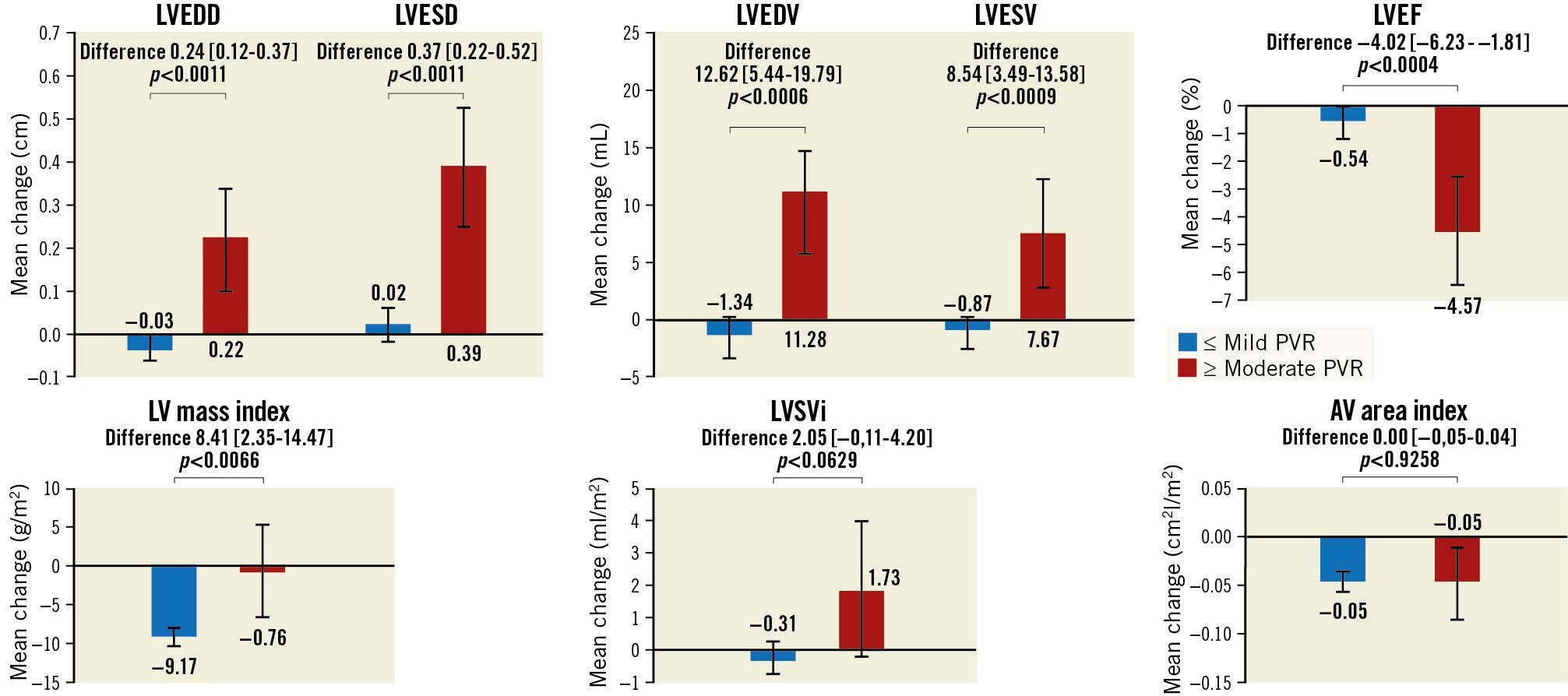
Central illustration. Adjusted echocardiographic changes between 30 days/discharge and two years post-TAVR based on PVR severity. The difference in various echocardiographic parameters between the 30 day/discharge and two-year echocardiogram are presented for ≥moderate PVR versus ≤mild PVR patients. Values are presented as least-square means (95% CI).
PVR severity and clinical outcomes
Rates of all-cause death were compared between patients with none/trace PVR, mild PVR, and ≥moderate PVR (Figure 1). While ≥moderate PVR patients had a significantly increased risk of all-cause death when compared with none/trace PVR patients at two years, those with mild PVR did not.
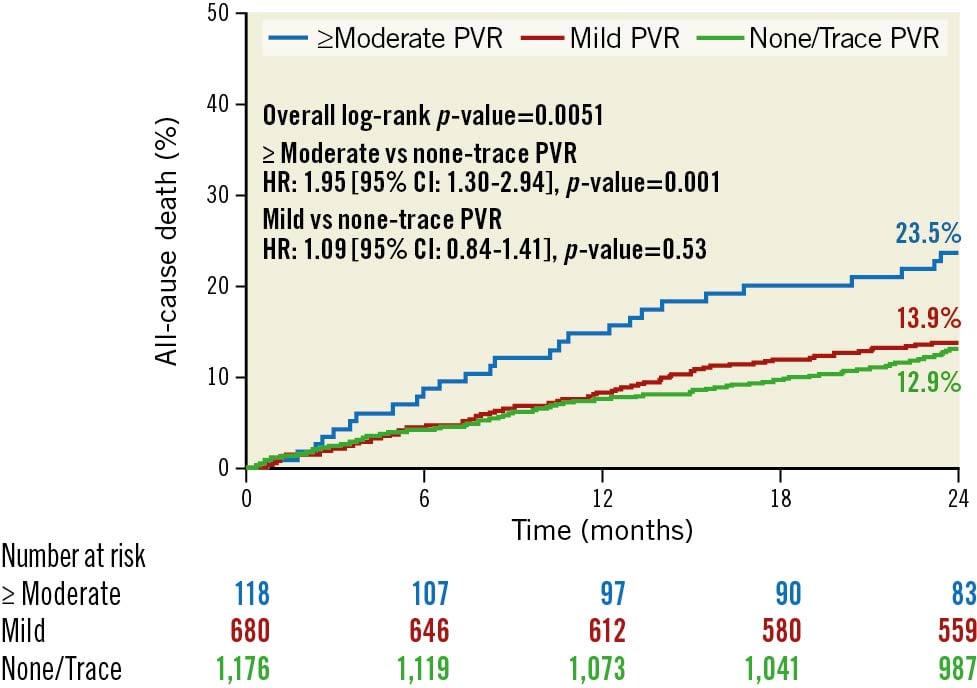
Figure 1. Association between PVR severity and all-cause death at two years. Kaplan-Meier survival curves for all-cause death are shown for ≥moderate PVR, mild PVR, and none/trace PVR. PVR: paravalvular regurgitation
When looking at other clinical outcomes, ≥moderate PVR patients had a more than twofold increase in risk of CV death, rehospitalisation, and reintervention when compared with £mild PVR patients at two years. There was no significant difference in stroke or non-CV death risk between the two groups (Figure 2). In multivariable analyses, ≥moderate PVR was again shown to be associated with an increased risk of all-cause death, CV death, rehospitalisation, and reintervention, but not with stroke when compared to £mild PVR at two years (Table 3). These results were consistent with propensity score-adjusted sensitivity analyses (Supplementary Table 4). The majority of rehospitalisations for both groups were due to congestive heart failure, and all reinterventions in the ≥moderate PVR group were due to non-structural dysfunction PVR (Supplementary Table 5, Supplementary Table 6). Subgroup analyses did not show any heterogeneity in effect of PVR severity on all-cause death at two years in any of the subgroups examined, which included patients who received the SAPIEN XT versus the SAPIEN 3 valve, patients with and without baseline moderate or severe AR, and patients with and without baseline LV ejection fractions >50% (Figure 3).
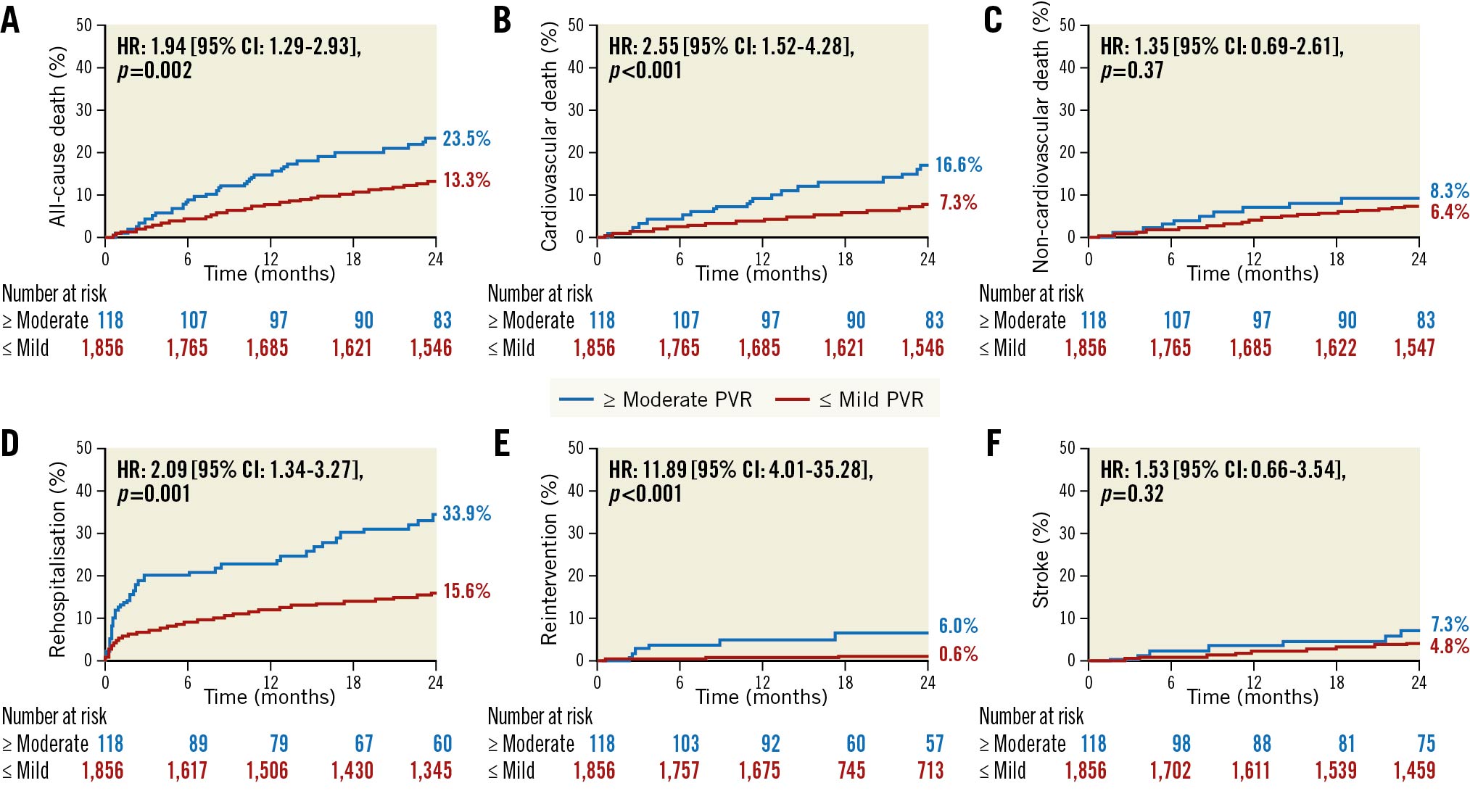
Figure 2. Association between PVR severity and clinical outcomes at two years. Kaplan-Meier survival curves are shown for ≥moderate PVR versus ≤mild PVR with regards to (A) all-cause death, (B) cardiovascular death, (C) non-cardiovascular death, (D) rehospitalisation, (E) reintervention, and (F) stroke. PVR: paravalvular regurgitation
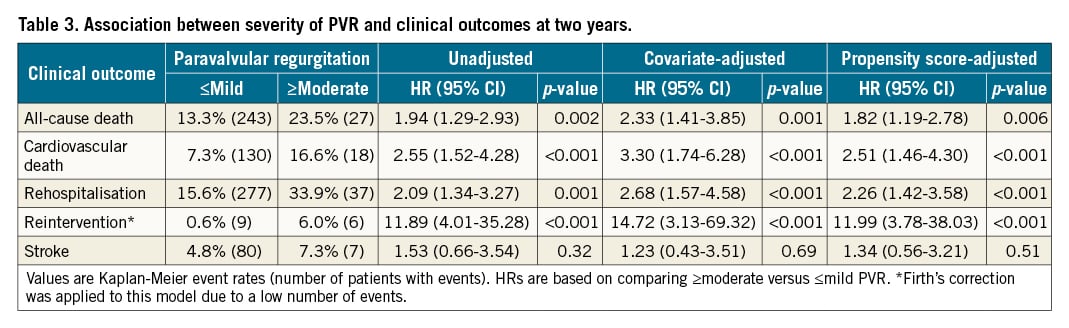
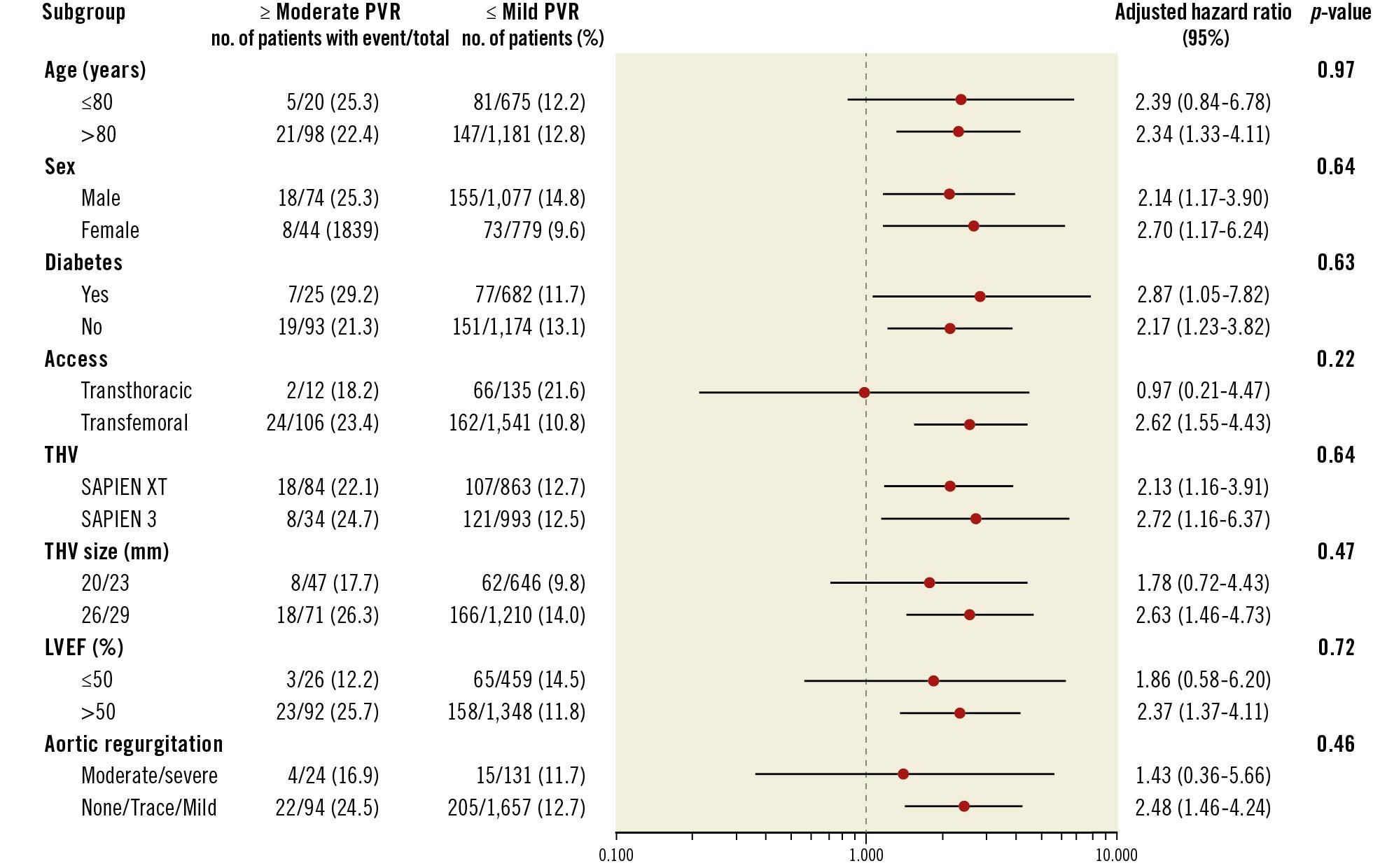
Figure 3. Subgroup analyses of the association between PVR severity and all-cause death or rehospitalisation at two years. Adjusted HRs and CIs are shown for the risk of all-cause death or rehospitalisation at two years in ≥moderate PVR versus ≤mild PVR patients among the subgroups depicted. LVEF: left ventricular ejection fraction; PVR: paravalvular regurgitation; THV: transcatheter heart valve
Landmark analyses showed that mild PVR patients had no significant increase in risk of all-cause death between one and two years when compared with none/trace PVR patients (Supplementary Figure 1). Moderate or worse PVR patients, however, continued to have a more than twofold increase in the risk of all-cause death, CV death, rehospitalisation, and reintervention between one and two years post-TAVR (Supplementary Figure 2). When all-cause death and rehospitalisation were combined as composite outcomes, landmark analyses at one year showed that ≥moderate PVR patients had a 2.88 times increased risk of the composite outcome (95% CI: 1.55-5.34; p-value<0.001). Moderate or worse PVR patients continued to have an increased risk of all-cause death or rehospitalisation between one and two years after adjustment for LV end-diastolic and LV end-systolic dimensions at one year (total effect: HR 3.18, 95% CI: 1.44-6.41; p-value=0.006; direct effect: HR 2.83, 95% CI: 1.21-5.84; p-value=0.009). The same was found when adjusting for the one-year LVEF (in addition to adjusting for LV end-diastolic and end-systolic dimensions) (total effect: HR 3.18, 95% CI: 1.44-6.41; p-value=0.006; direct effect: HR 2.84, 95% CI: 1.25-5.78; p-value=0.009).
Discussion
This is the largest study to investigate the association between PVR severity post-TAVR and clinical outcomes and the mechanisms by which it may occur. Among 1,974 patients with severe symptomatic AS at intermediate surgical risk treated with TAVR, the major findings were the following: 1) Moderate or worse PVR, but not mild PVR, was independently associated with a higher adjusted risk of not only all-cause and CV death, but also rehospitalisation and reintervention at two years. 2) Patients with ≥moderate PVR who survived to one year post-TAVR continued to have an increased risk of all-cause death, CV death, rehospitalisation, and reintervention at two years. 3) Moderate or worse PVR was also associated with adverse cardiac remodelling changes in the form of larger LV dimensions and volumes, lower LVEF, and blunted LV mass index regression at one year that continued to progress up to two years post-TAVR. 4) Adverse remodelling changes in LV dimensions and LVEF may partially mediate how ≥moderate PVR leads to worse clinical outcomes. Collectively, our findings highlight the deleterious impact that ≥moderate PVR has on LV remodelling, and that those LV remodelling changes may be partial mediators in the pathway between ≥moderate PVR and poor clinical outcomes.
Mild PVR and outcomes
The association of ≥moderate PVR with increased risk of mortality has been well-established for both older and current valve generations123. Whether mild PVR is associated with increased mortality risk, however, is more controversial with some studies of high surgical risk patients receiving older generations of heart valves having found mild PVR to be associated with increased mortality11213. In this study of close to 2,000 intermediate-risk patients who received TAVR, over half of whom received the SAPIEN 3 valve, while ≥moderate PVR was associated with an increased risk of all-cause mortality at two years, mild PVR was not. While our results add to the growing body of evidence that mild PVR is not associated with increased mortality, we cannot exclude that mild PVR may have an impact on clinical outcomes beyond two years; further follow-up is needed.
Echocardiographic parameters and clinical outcomes
Despite similar baseline parameters, at one year, ≥moderate PVR patients had larger LV dimensions and volumes, lower LVEFs, and blunted LV mass regression. Notably, changes in LV dimensions and LVEF were found to partially mediate the effects of ≥moderate PVR on all-cause death and rehospitalisation. These findings were driven mainly by patients with moderate PVR as only 9 of the 118 patients in the ≥moderate PVR group had severe PVR.
Adverse cardiac remodelling resulting in a more dilated left ventricle or a reduced EF has long been shown to be associated with worse outcomes14. In the post-TAVR population, previous studies have also shown that a decrease or lack of improvement in LVEF, as well as larger LV dimensions when compared with pre-TAVR measurements, are associated with increased mortality risk151617. It has been postulated that in PVR patients, the sudden change from pressure to volume overload is not well tolerated and leads to adverse cardiac remodelling17. In line with this, a previous study showed that patients who developed PVR post-TAVR did worse if they did not have pre-TAVR AR compared to those who did9. While in this study, we did not find any difference in outcomes in ≥moderate PVR patients based on pre-TAVR AR severity, our findings are consistent with previous studies that have shown PVR to be associated with adverse cardiac remodelling changes181517.
Our study is the first to show that the adverse cardiac remodelling changes associated with moderate PVR are also associated with the worse outcomes of patients experiencing moderate PVR . However, changes in LV dimensions and LVEF were found to only partially explain the effects of PVR on outcomes. Further studies are needed to elucidate the other contributing factors.
Clinical implications
Given that ≥moderate PVR patients had an almost twofold increase in risk of all-cause death, an over twofold increase in risk of CV death and rehospitalisation, and an over 11-fold increase in risk of reintervention, all efforts should be made to avoid ≥moderate PVR at the time of TAVR. Clearly the use of the newer-generation balloon-expandable valve is associated with a lower incidence of significant PVR as the majority of patients with ≥moderate PVR received the SAPIEN XT valve. However, intervening at the time of valve implantation requires an accurate assessment of AR severity, which would include comprehensive intraprocedural or periprocedural imaging.
The adverse cardiac remodelling changes that were found, in part, to facilitate ≥moderate PVR and poor outcomes in this study could possibly be a target for medical therapy after TAVR. Angiotensin-converting enzyme inhibitors (ACEis) and angiotensin receptor blockers (ARBs), for example, have been shown to lead to improvements in cardiac remodelling and clinical outcomes in AS patients18. A recent study showed that treatment with ACEis/ARBs in TAVR patients was also associated with improved outcomes; a randomised clinical trial on the impact of ACEi/ARB treatment on clinical outcomes and ventricular remodelling in TAVR patients is underway1920. Treatment with medications such as ACEis and ARBs that lead to improvement in cardiac remodelling may improve outcomes in patients with ≥moderate PVR after TAVR. However, further studies are needed.
Limitations
This is a post hoc analysis of the PARTNER 2A trial and S3i registry, which were not designed or powered to examine outcomes based on PVR severity. The findings of this study should therefore be considered hypothesis-generating. Also, while over half of the patients in this study received the SAPIEN 3 valve, the majority of patients with ≥moderate PVR received the SAPIEN XT valve. The results may therefore have been driven mainly by patients who received the SAPIEN XT valve, which is an older-generation valve. Residual confounding based on the type of valve received is possible, though statistical models were stratified by study (and therefore valve type) in order to avoid this. While we attempted to adjust for all possible confounders, it is still possible that there is residual confounding based on inherent differences between the ≥moderate PVR and ≤mild PVR groups. Also, data such as pre-TAVR computed tomography imaging and cardiac medications that would have been interesting to include in the analyses were, unfortunately, unavailable.
Conclusions
Moderate or worse PVR, but not mild PVR, post-TAVR is associated with an increased risk of all-cause and CV death, rehospitalisation, and reintervention at two years. Moderate or worse PVR is also associated with adverse cardiac remodelling changes, and larger LV dimensions and lower LVEF may partially mediate how PVR leads to worse outcomes. Our findings highlight not only the importance of avoiding PVR post-TAVR, but also the mechanism behind why ≥moderate PVR is associated with worse outcomes, providing potential targets for medical therapies.
Impact on daily practice
Moderate or worse PVR post-TAVR is associated with worse clinical outcomes at two years; adverse cardiac remodelling changes are part of the mechanism by which that happens.
Funding
This work was supported by the Cardiovascular Research Foundation, New York, NY, USA, which received funding from Edwards Lifesciences for statistical analysis and editorial/administrative support in relation to this manuscript. The PARTNER 2 trial was also sponsored by Edwards Lifesciences.
Conflict of interest statement
K. Chau reports support from an institutional grant by the National Institutes of Health/ National Heart, Lung, and Blood Institute to Columbia University Irving Medical Center (T32 HL007854). R. Hahn reports speaker fees from Boston Scientific, Baylis Medical, Edwards Lifesciences and Medtronic, consulting for Abbott Structural, Edwards Lifesciences, Gore & Associates, Medtronic, NaviGate, and Philips Healthcare, non-financial support from 3mensio, Equity with NaviGate, and is the Chief Scientific Officer for the Echocardiography Core Laboratory at the Cardiovascular Research Foundation for multiple industry-sponsored trials, for which she receives no direct industry compensation. S. Kodali has received consulting fees from Edwards Lifesciences, Medtronic, Claret Medical, Merrill Lifesciences, BioTrace Medical, and Micro Interventional Devices, has served on advisory boards for Thubrikar Aortic Valve, Dura Biotech, Abbott Vascular, Paieon Medical, and St. Jude Medical, and owns equity in Thubrikar Aortic Valve, Dura Biotech, BioTrace Medical, and Micro Interventional Devices. W. Jaber has echocardiography core laboratory contracts with Edwards Lifesciences (no direct compensation). V. Thourani has received research support from Edwards Lifesciences, Boston Scientific, and JenaValve, and consulting fees from Edwards Lifesciences, Boston Scientific, Abbott, Gore Vascular, and JenaValve. P. Pibarot has research contracts with Edwards Lifesciences for echo core lab analyses with no personal compensation. M. Leon has received institutional research funding from Edwards Lifesciences, Medtronic, Boston Scientific, and Abbott, and has consulted for Medtronic, Boston Scientific, Gore Medical, Meril Lifesciences, and Abbott. He is a member of the Executive Committee for the PARTNER Trials (no direct compensation). The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

