
Abstract
Aims: Aortic regurgitation (AR) is common after transcatheter aortic valve implantation (TAVI). Intraprocedural assessment of AR relies on aortic root angiography. Cardiac magnetic resonance (CMR) phase-contrast mapping of the ascending aorta provides accurate AR quantification. This study evaluated the accuracy of AR grading by aortic root angiography after TAVI in comparison to CMR phase-contrast velocity mapping.
Methods and results: In 69 patients with TAVI for severe aortic stenosis, post-procedural AR was determined by aortic root angiography with visual assessment according to the Sellers classification and by CMR using phase-contrast velocity mapping for analysis of AR volume and fraction. Spearman’s correlation coefficient showed a moderate correlation between angiographic analysis of AR grade and CMR-derived AR volume (r=0.41; p<0.01) as well as AR fraction (r=0.42; p<0.01). There was significant overlap between the angiographic Sellers classes compared to CMR-derived AR fractions. Aortic root angiography with cut-off Sellers grade ≥2 had a sensitivity of 71% and a specificity of 98% to detect AR graded as moderate to severe or severe as defined by CMR.
Conclusions: There is only a moderate correlation between aortic root angiography and CMR in the classification of AR severity after TAVI. Alternative imaging including multimodality imaging as well as haemodynamic analysis should therefore be considered for intraprocedural AR assessment and guidance of TAVI procedure in cases of uncertainty in AR grading.
Introduction
Transcatheter aortic valve implantation (TAVI) has become an important therapeutic option for high-risk or non-operable patients with severe aortic valve stenosis1-5. A common limitation after the TAVI procedure is aortic regurgitation (AR) due to paravalvular leakage. Paravalvular AR occurs with a higher frequency after TAVI than after surgical aortic valve replacement6,7. Several studies have shown a relationship between AR severity and short- and long-term clinical outcome, with reduced survival rates in patients with higher grades of AR7-12.
Besides optimal planning of the TAVI procedure with a multimodal imaging strategy13, accurate intraprocedural assessment of AR severity is crucial to guide the TAVI procedure and to achieve optimal implantation results. The intraprocedural assessment of AR mainly depends on aortic root angiography. Although it is an established tool for the assessment of AR, it is limited by the subjective visual assessment of contrast agent backflow from the aorta into the left ventricle14. Cardiac magnetic resonance (CMR) imaging is the gold standard method for the assessment of LV volumes and has also gained clinical acceptance in the evaluation of cardiac valves15,16. It allows for a reliable, highly reproducible quantification of AR by flow-sensitive phase-contrast velocity mapping of the ascending aorta independent of the functional and anatomical nature of AR17,18.
This study sought to evaluate the accuracy of aortic root angiography for AR assessment after TAVI procedure in comparison to CMR phase-contrast velocity mapping as reference standard.
Methods
STUDY DESIGN AND PATIENT POPULATION
Between January 2011 and July 2012, 96 patients underwent transapical or transfemoral TAVI for severe symptomatic calcified native aortic valve stenosis at our hospital. Patients had to have an aortic valve area <1 cm2, and an aortic valve annulus diameter of 18 to 27 mm. The presence of a bicuspid aortic valve as determined by transoesophageal echocardiography was an exclusion criterion for TAVI. Twenty-seven patients were excluded from this study due to typical contraindications for a CMR examination. One patient had to be excluded from CMR due to cranial metallic clips. Three patients resisted CMR due to claustrophobia. Twenty patients had to be excluded from CMR imaging due to pacemaker implantation. Five patients had pre-procedural pacemaker implants and 15 patients had post-TAVI pacemaker implantation due to new AV block. Three patients were excluded due to prolonged intensive care unit stay. The remaining 69 patients formed the study group (Figure 1). Patients with atrial fibrillation were not excluded. Thirty-one patients (45%) underwent transfemoral TAVI using the CoreValve Revalving® System (Medtronic, Minneapolis, MN, USA) and 38 patients (55%) underwent transaortic TAVI using the Edwards SAPIEN XT valve (Edwards Lifesciences, Irvine, CA, USA). This study was approved by the ethical committee of the University of Aachen and all patients gave written informed consent.
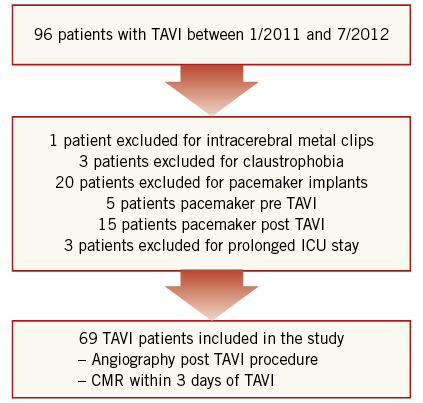
Figure 1. Flow chart of patient inclusion and exclusion into the study. ICU: intensive care unit
TAVI PROCEDURE
Patients were referred for TAVI after a senior cardiologist and a senior cardiac surgeon had reached a consensus that surgical replacement would be associated with either high or prohibitive risks. Details of the devices and the technical aspects of the procedure have been published previously1-3. To reduce AR detected immediately after implantation of the valve prosthesis, seven patients in the Edwards SAPIEN XT group and five patients in the CoreValve Revalving group underwent post-dilatation. In addition, a snaring technique to reposition the CoreValve prosthesis to a more upwards position was applied in two patients.
AORTIC ROOT ANGIOGRAPHY
At the end of the TAVI procedure final aortic root angiography was performed after withdrawal of all LV catheters. When the self-expanding CoreValve prosthesis was implanted, angiography was performed at least 10 min after deployment of the implant to allow adequate expansion. Using a pigtail catheter, 30 ml of contrast agent was injected at a rate of 15 ml/sec in the proximal ascending aorta directly above the implanted aortic valve. Aortic angiography was performed in right anterior oblique (RAO) 30° projection with a frame rate of 15/sec (Figure 2). Aortic valve regurgitation was assessed offline by visual grading of the severity of contrast agent backflow from the aorta into the left ventricle according to the Sellers classification (grade 0: absence of AR, grade 1: small amount of contrast entering the LV during diastole without filling the entire cavity and clearing with each cardiac cycle, grade 2: contrast filling of the entire LV in diastole, but with less density as compared to contrast opacification of the ascending aorta, grade 3: contrast filling of the entire LV in diastole equal in density to the contrast opacification of the ascending aorta, grade 4: contrast filling of the entire LV in diastole on the first beat with greater density as compared to the contrast opacification of the ascending aorta). Grading was performed by an experienced interventional cardiologist (RH) with more than 20 years of experience in invasive cardiology and more than five years of experience in performing TAVI procedures (>500 procedures). The analysis of angiograms was blinded to clinical outcomes of the patients.
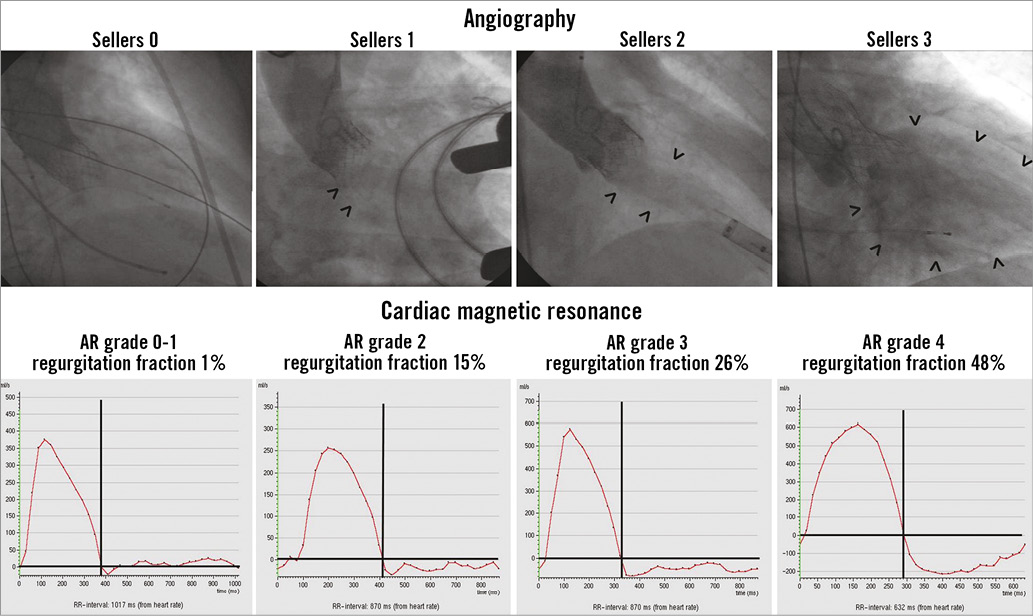
Figure 2. Angiography and cardiac magnetic resonance analysis. Upper row: angiographic analysis of aortic regurgitation at the end of the TAVI procedure in right anterior oblique (RAO) 30° projection (arrows indicate contrast agent backflow from the aorta into the left ventricle). Lower row: CMR phase-contrast velocity mapping analysis: diagram showing flow rate over one heart cycle in the ascending aorta (positive values indicating forward flow and negative values indicating backward flow/aortic regurgitation).
CARDIAC MAGNETIC RESONANCE
CMR was carried out within three days after the TAVI procedure on a 1.5 Tesla MRI scanner (Philips Achieva; Philips Healthcare, Best, The Netherlands) with the patient in supine position. A five-element cardiac synergy coil was used for signal reception and a vector ECG for cardiac synchronisation. The study was performed in short repetitive end-expiratory breath holding. After the survey and planning scans, steady-state free precession (SSFP) cine imaging (retrospective gating; 35 phases per cardiac cycle; sensitivity encoding [SENSE] factor: 1.4; spatial resolution 1.4×1.4×8 mm; TR: 3.1 ms, TE: 1.6 ms, flip angle 55°) in three standard long-axis (2-, 4-, and 3-chamber view) and LVOT geometries, as well as in short-axis orientation with full ventricular coverage, was performed. Based on the survey, and the cine 3-chamber and LVOT images, a breath hold through-plane velocity-encoded phase-contrast sequence (retrospective gating; 35 phases per cardiac cycle; sensitivity encoding [SENSE] factor: 2.0; spatial resolution 1.4×1.4×10 mm; TR/TE/flip angle: 3.9 ms/2.4 ms/15°; breath hold duration 12-16 seconds) was planned, orthogonal to the ascending aorta just above the cage of the TAVI prosthesis (Figure 3). Maximum velocity encoding was set to 2.0 m/s and individually adapted (increased by 0.5 m/s steps) to avoid aliasing if necessary. The phase-contrast velocity-mapping-based measurement was repeated three times. In the first 20 patients with the Edwards SAPIEN prosthesis, the phase-contrast measurements were also performed in a transversal plane at the level of the mid ascending aorta.
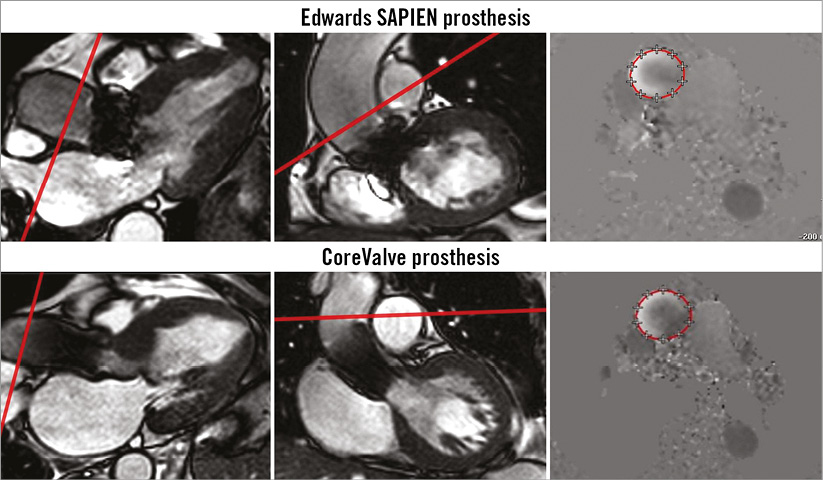
Figure 3. Cardiac magnetic resonance planning of phase-contrast velocity mapping geometry for the Edwards SAPIEN XT (upper row) and the CoreValve® prosthesis (lower row) using 3-chamber and left ventricular outflow tract cine images after TAVI with the red line indicating phase-contrast velocity geometry. The geometry was controlled on non-angulated survey images and adapted if necessary.
CMR data were analysed offline on a dedicated MR workstation (Extended Workspace; Philips Healthcare) by blinded readers with more than three years of CMR experience. LV volumes and EF were assessed from the short-axis stack according to the disc summation method (Simpson’s rule). In the flow-sensitive phase-contrast images, the cross-sectional area of the ascending aorta was tracked over the heart cycle by computer-assisted contour recognition and manually corrected if necessary. Aortic forward and backward flows were then calculated by the software (Figure 2). Aortic regurgitation volume was determined as diastolic aortic backward flow. Aortic regurgitation fraction was calculated as division of aortic backward flow by aortic forward flow. Finally, the mean of the three phase-contrast velocity mapping measurements was calculated and used for this study. In agreement with previous study results, CMR-RF ≤8% was graded as none or mild (Grade 0 or I), 9-18% as moderate (Grade II), 19-29% as moderate to severe (Grade III) and ≥30% as severe (Grade IV)19.
OBSERVER VARIABILITY
Interobserver and intraobserver variability in the angiographic grading of AR severity as well as definition of AR regurgitation volume and fraction by CMR was analysed on the first 20 patients. For each imaging modality a second observer independently evaluated aortic regurgitation to determine interobserver variability. Furthermore, the same studies were re-evaluated by observer one at least four weeks apart to determine the intraobserver variability.
STATISTICAL ANALYSIS
Statistical analysis was performed with the use of SPSS software version 21.0 (IBM Corp., Armonk, NY, USA). Categorical data were presented as frequencies and compared with the Pearson chi-square test. Continuous data were presented as mean±SD. For correlation between angiographic Sellers classification and CMR-derived AR volume, AR fraction and AR grade, the Spearman correlation coefficient (rho) was calculated (for correlation of angiographic and CMR-derived AR grades, angiographic Sellers grade 0 and 1 were merged into one group). Furthermore, box plot diagrams were generated. The interobserver and intraobserver variability for the continuous data obtained by CMR was determined by calculation of the mean of the signed difference between two measurements divided by the mean of both measurements and subsequent calculation of the standard deviation of these data. Additionally, for analysis of intraobserver and interobserver agreement on CMR-derived AR volume and AR fraction, as well as for angiographic grading of AR severity, the interclass correlation coefficient (ICC) was determined. Weighted kappa values were calculated for intraobserver and interobserver variability analysis in the angiographic assessment of aortic regurgitation severity. For comparison of phase-contrast-based measurements at different aortic levels, a t-test for paired samples was used. Additionally, to analyse the reproducibility of the CMR phase-contrast measurements, the standard deviation among the three CMR phase-contrast measurements per patient on aortic regurgitation volume and regurgitant fraction was averaged over all patients as well as for patients with sinus rhythm and patients with atrial fibrillation. A p<0.05 was considered statistically significant.
Results
Patient characteristics of all 69 patients included in the study are given in Table 1. Fourteen patients (20%) had atrial fibrillation.
QUANTIFICATION OF AORTIC REGURGITATION BY CMR
CMR-derived values are given in Table 2 (examples in Figure 2). Mean aortic regurgitation volume after TAVI as determined by CMR was 9.2±9.4 ml, and mean aortic regurgitation fraction was 13.0±9.6%. Based on CMR regurgitation fraction, 26 patients (38%) were graded as having none or mild AR, 30 patients (43%) were graded as having moderate AR, eight patients (12%) were graded as having moderate to severe AR and five patients (7%) were graded has having severe AR. Comparison of phase-contrast-derived measurements from two different imaging planes (just above the cage of the TAVI prosthesis vs. mid ascending aorta level) performed in the first 20 patients with the SAPIEN valve revealed no significant differences in stroke volume (71.3±22.4 vs. 71.4±22.4 ml, p=0.732), in regurgitant volume (9.2±9.1 vs. 9.2±8.4 ml, p=0.991) or in regurgitant fraction (11.9±8.1 vs. 11.9±7.7%, p=0.965), respectively, between the two imaging planes.
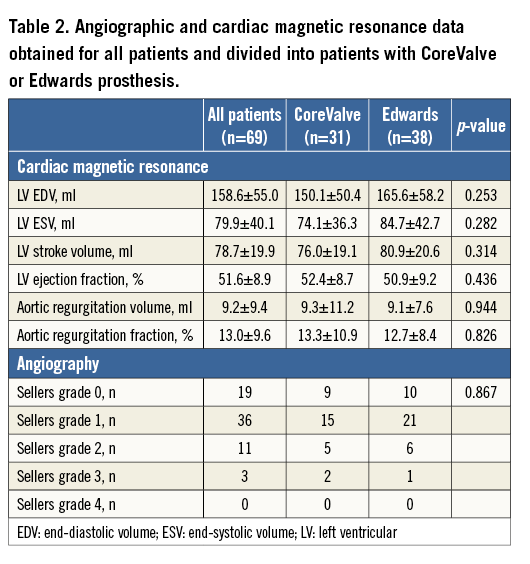
There was no significant difference in regurgitant volume or regurgitant fraction as defined by CMR between patients with an Edwards SAPIEN implant and those with a CoreValve implant (Table 2).
GRADING OF AORTIC REGURGITATION BY ANGIOGRAPHY
According to the Sellers classification, aortic regurgitation was graded as absent or mild (Sellers grade 0 and 1) in 55 patients (79.8%). In 11 patients (15.9%), AR was graded as Sellers 2, in three patients (4.3%) it was graded as Sellers 3, and in no patients it was graded as Sellers 4 (Figure 2). There was no significant difference in regurgitant grade between patients with an Edwards SAPIEN implant and those with a CoreValve implant (Table 2).
CORRELATION OF ANGIOGRAPHY AND CMR
Considering the angiographic assessment of AR severity, there was extensive overlap among the different Sellers classes in AR fractions as well as AR volumes as defined by CMR (Table 3). The box plot diagram (Figure 4) demonstrates the broad overlap of Sellers classes in relation to AR fractions and volumes determined by CMR. Angiographic analysis had a sensitivity of 71% and a specificity of 98% if a Sellers grade ≥2 was used as cut-off, and a sensitivity of 18% and a specificity of 98% if a Sellers grade ≥3 was used as cut-off to detect an AR which was defined as at least moderate to severe AR by CMR. Spearman correlation coefficient showed a moderate positive correlation between angiographic determination of AR grade and CMR-derived AR volume and AR fraction (r=0.41 [p=0.001] and r=0.42 [p<0.001], respectively) (Table 3). For all patients, the correlation between angiographic Sellers grade and CMR-derived AR grade was r=0.49 (p<0.001). In a subgroup analysis of patients with atrial fibrillation (n=14 patients), the correlation between AR volume and angiographic Sellers grade was r=0.47 (p=0.092), and the correlation between AR fraction and angiographic Sellers grade was r=0.53 (p=0.051).
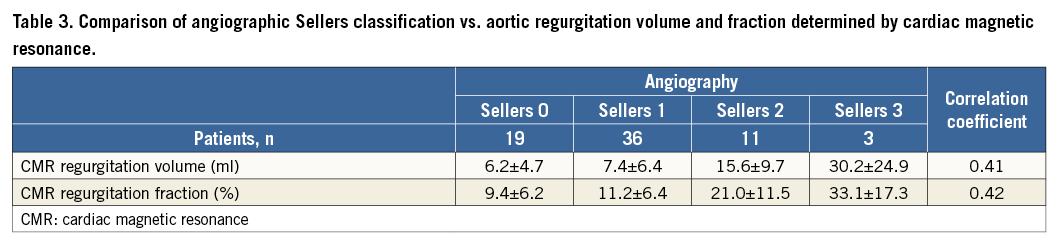
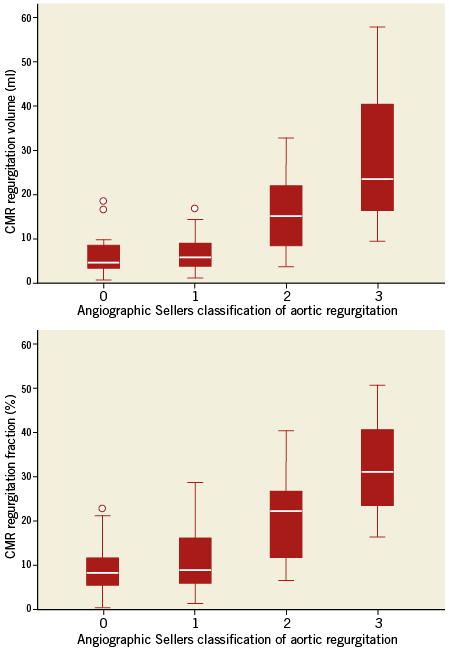
Figure 4. Box plot diagrams showing the association between angiographic Sellers classification of aortic regurgitation and CMR-derived aortic regurgitation volume and fraction.
Observer variability
Intraobserver and interobserver variability for CMR and angiographic AR assessment was determined. Considering the mean of the three CMR measurements per patient, intraobserver variability for the regurgitation volume was 2.2±1.9 ml (ICC: 0.999) and intraobserver variability for the regurgitation fraction was 1.5±1.1% (ICC: 0.999). Interobserver variability on CMR-determined regurgitation volume was 1.5±1.4 ml (ICC: 0.999), and on regurgitation fraction it was 1.5±0.8% (ICC: 0.999). For angiographic grading of AR severity the analysis of intraobserver agreement demonstrated an ICC of 0.768, and the analysis of interobserver agreement demonstrated an ICC of 0.511. Considering the kappa statistics, the kappa value for intraobserver variability analysis in the angiographic assessment of AR severity was 0.676 and the kappa value for interobserver variability analysis was 0.450.
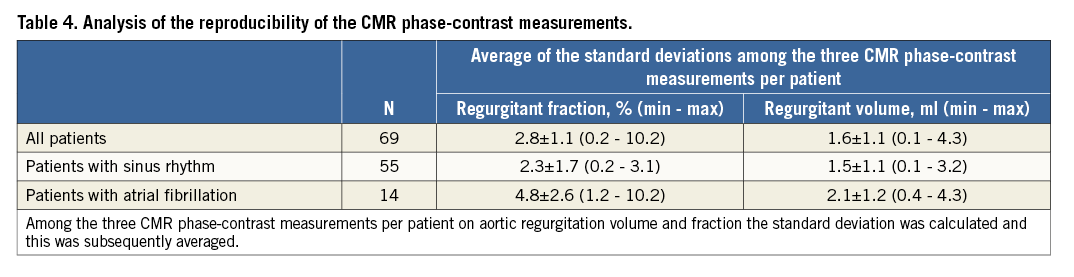
REPRODUCIBILITY OF CMR PHASE-CONTRAST MEASUREMENTS
To determine reproducibility of CMR-derived phase-contrast measurements for all patients, for patients in sinus rhythm and for patients in atrial fibrillation, the mean standard deviation among the three measurements per patient in the analysis of regurgitant volume and regurgitant fraction was determined (Table 4).
Discussion
The major findings of this study are as follows. 1) In the majority of patients there was only mild or mild to moderate AR after TAVI as defined by CMR phase-contrast velocity mapping. 2) The accuracy of aortic root angiography to detect moderate to severe or severe AR as defined by CMR phase-contrast velocity mapping is acceptable if a Sellers grade 2 is used as cut-off. 3) Angiographic grading of AR severity is characterised by significant overlap among the Sellers classes in relation to regurgitant volume and fraction as determined by CMR. 4) Intraobserver and interobserver variability on assessment of AR after TAVI is considerable using the angiographic Sellers classification, and very low using CMR phase-contrast velocity mapping of the ascending aorta.
AORTIC REGURGITATION AFTER TAVI
Paravalvular leak is more frequent after TAVI compared to conventional surgical aortic valve replacement. Reported rates of moderate to severe paravalvular leak after TAVI are in the range of 15-20%7,12,20-22. These rates are reported despite additional manoeuvres having been performed during the procedure to reduce paravalvular leak such as post-dilatation, snare traction of the implant or valve-in-valve implantation12. Several studies have suggested a negative impact of paravalvular leak on short- and long-term prognosis after TAVI11,12. However, there is a lack of a standardised quantitative method of assessing paravalvular leakage. For post-interventional analysis, echocardiography has been used. Valve Academic Research Consortium criteria have been defined to standardise the assessment of paravalvular regurgitation after TAVI using echocardiography23; however, during the interventional procedure aortic root angiography with injection of contrast is the main modality for assessment.
AORTIC ROOT ANGIOGRAPHY
Aortic root angiography is a widely accepted modality for the evaluation of AR. It can be performed easily and quickly during the TAVI procedure to assess AR. It provides important information, which may result in additional manoeuvres during the intervention if significant AR is detected. However, analysis is based on a subjective visual assessment of contrast regurgitation. Thus, it provides a tool for qualitative assessment and not for accurate quantitative analysis. Furthermore, analysis of contrast backflow is usually based on only one two-dimensional view, which may not be in the direction of the regurgitation jet and therefore may not allow adequate assessment of AR severity. Normally, the Sellers criteria are used to grade AR based on angiography14. Significant interobserver variability in the analysis of angiographic imaging has been reported24,25. In case of TAVI procedures, important therapeutic and prognostic consequences are related to the subjective analysis of AR.
However, the variability and accuracy of AR assessment after TAVI based on aortic root angiography related to a quantitative analysis tool has not been evaluated so far. In a small series of 16 post-TAVI patients, Sherif et al compared AR grading by CMR to AR grading by aortic root angiography26. They reported a good correlation between both methods. This result was different from our findings with only moderate correlation in AR grading between the two methods. This difference may be due to a different grading system used for the CMR-based AR grading, with smaller ranges in the AR fraction for each AR grade used in our study compared to the study by Sherif et al. The CMR-based grading system applied in our study relates to previously applied and recommended grading systems19. However, the comparison of AR grading by angiography with the quantitative analysis of AR fraction and volume also showed only a moderate correlation in our study. Considering the robust and relatively user-independent, semi-automated quantitative analysis of AR based on CMR phase-contrast velocity mapping15,16, some imprecision of AR grading by aortic root angiography is indicated by the results. The very low interobserver and intraobserver variability in the analysis of AR severity as defined in this study supports the strength of CMR.
The accuracy of Sellers AR grading to detect AR defined by CMR phase-contrast velocity mapping as being moderate to severe or severe was acceptable with a sensitivity of 71% and a specificity of 98%. Importantly, this accuracy was obtained if a Sellers grading of 2 or more was considered as important AR in the angiographic analysis. Considering a Sellers grading of 3 or more, the sensitivity to detect AR moderate to severe or severe by CMR dropped to 18%. Thus, already a Sellers grade 2 AR by aortic root angiography indicates moderate to severe or severe AR as defined by CMR. However, a considerable overlap among the Sellers AR grades was found considering CMR regurgitation volumes and fractions. Thus, on an individual patient basis, aortic root angiography has limitations in the analysis of AR severity. This should be considered when severity of AR after TAVI is reported or the severity of AR after TAVI is compared between different prosthesis types based on aortic root angiography. Therefore, additional tools to assess AR severity after TAVI should be considered even in the catheterisation laboratory to obtain a more accurate analysis of AR severity. These tools may be based on analysis of haemodynamics or additional imaging modalities27. Two-dimensional (2D) and three-dimensional (3D) echocardiography provide quantitative tools for analysis of AR severity, which are easily applicable in the catheterisation laboratory28,29. However, in the echocardiographic analysis of regurgitant jets, limitations due to acoustic shadowing by the prosthesis or aortic calcification have to be considered. Thus, multimodality imaging may be the ideal tool to determine AR severity accurately during the procedure. After the procedure, CMR may be considered in case of clinically suspected relevant AR in spite of non-significant AR defined by angiography and echocardiography.
Limitations
While angiography was performed immediately after the TAVI procedure, CMR was performed within three days after the procedure. Different haemodynamic conditions might have slightly altered the severity of aortic regurgitation. The imaging modalities were compared for quantification of paravalvular AR but no information about the impact of AR severity on outcome at follow-up was obtained in this study. CMR and angiography do not allow distinguishing between paravalvular and valvular AR. CMR has gained broad clinical acceptance in the evaluation of native cardiac valves15,16, although there are several known pitfalls due to through-plane motion and turbulent flow. Less evidence exists for its use in aortic valve prostheses. However, considering the tricuspid structure of TAVI prostheses, which is similar to the native valve, and the fact that TAVI prostheses are typically not implanted in patients with aortic dilation, a similar accuracy and reproducibility of CMR-derived AR quantification may be expected in TAVI patients.
Analysis of AR severity by phase-contrast imaging CMR was performed just above the TAVI prosthesis. Considering the different configuration of the CoreValve and the Edwards prostheses, the phase-contrast geometry was placed at slightly different levels of the ascending aorta, with the imaging plane between the sinotubular junction and the mid ascending aorta for the Edwards prosthesis, and at the mid ascending aorta level for the CoreValve prosthesis. A previous study has demonstrated a variation of AR severity by phase-contrast imaging CMR depending on the site of the flow measurements in the ascending aorta, with decreasing regurgitation fraction at increasing distance from the aortic valve19. A difference of approximately 10% between analysis at the sinotubular junction and the mid ascending aorta has been demonstrated. However, the spatial difference between the two imaging planes used in this study was significantly less. Thus, differences resulting from the different measurement points are minimal as confirmed by analysis of AR severity at both points in 20 patients after implantation of the Edwards SAPIEN prosthesis. Therefore, an imaging plane as close as possible to the aortic valve has been selected in all patients. Fourteen of the 69 patients (20%) were in atrial fibrillation. Reproducibility of AR analysis by CMR was found to be slightly worse in these patients than in patients with sinus rhythm. This might have had an impact on the overall association between CMR and angiographic findings. This study was limited to the analysis of AR based on CMR and angiography. Systematic echocardiographic data were not available for inclusion in the analysis. Furthermore, no outcome data are available to allow conclusions on the association between AR severity by CMR or angiography and subsequent patient outcome.
Conclusion
Angiographic grading of AR severity after TAVI is limited by inaccuracies when CMR-defined regurgitation volume and regurgitation fraction are used for comparison. Alternative imaging including multimodality imaging as well as haemodynamic analysis should therefore be considered for intraprocedural AR assessment and guidance of TAVI procedure. This relates in particular to cases with discordance between the clinical status of the patient and the angiographic grading of AR severity.
| Impact on daily practice Aortic regurgitation after transcatheter aortic valve implantation is a common limitation. Aortic root angiography is the commonly applied technique for the grading of aortic regurgitation severity during the procedure and for guidance of additional procedural steps. However, caution should be applied regarding its accuracy for quantification. Comparison with cardiac magnetic resonance demonstrates considerable limitations. Thus, alternative imaging including multimodality imaging and haemodynamic analysis should be considered for assessment of aortic regurgitation severity and guidance during the TAVI procedure. |
Conflict of interest statement
The authors have no conflicts of interest to declare.

