- CoreValve
- magnetic resonance
- aortic regurgitation
Abstract
Aims: Echocardiography may underestimate the degree of paravalvular aortic regurgitation (AR) after transcatheter aortic valve implantation (TAVI) using the Medtronic CoreValve bioprosthesis due to inherent limitations of ultrasound imaging in the evaluation of implanted cardiac prostheses. We aimed to evaluate the accuracy and feasibility of cardiovascular magnetic resonance (CMR) in quantifying regurgitant volume (RV) and regurgitant fraction (RF) in patients treated with this bioprosthesis for severe calcific aortic stenosis, and to compare the results with echocardiography and aortography.
Methods and results: This study included 16 patients with a mean age of 78.7 years (eight women, eight men) who underwent successful TAVI using Medtronic CoreValve bioprosthesis. AR was evaluated by CMR, echocardiography, and aortography. Angiography was performed immediately after valve implantation. CMR and echocardiography were performed four weeks after valve implantation. There was a highly significant correlation between the CMR-derived and the angiographically-estimated degree of AR (r=0.86, p<0.001). On the other hand, there was only a limited correlation between CMR and echocardiography (r=0.374, p=0.15) as well as angiography and echocardiography (r=0.319, p=0.23) regarding the degree of AR. The weighted kappa for agreement between echocardiography and angiography was 0.14, for agreement between echocardiography and CMR 0.20, and for agreement between angiography and CMR 0.72. Echocardiography underestimated AR by one degree compared to CMR in five patients and 2 degrees in two patients; in six of these, the degree of AR obtained by CMR was similar to angiography.
Conclusions: In patients undergoing TAVI, comparisons between purely quantitative measurements of AR by CMR and qualitative assessment by angiography showed better correlations than those with echocardiography. This suggests that echocardiography may underestimate the degree of AR and CMR in these circumstances has a great potential in reliably measuring the severity of AR in a quantitative manner.
Abbreviations
TTE transthoracic echocardiography
AR aortic regurgitation
TAVI transcatheter aortic valve implantation
CMR cardiovascular magnetic resonance
RF regurgitant fraction
RV regurgitant volume
LV left ventricular
Introduction
Echocardiography is the standard tool to assess the severity of aortic regurgitation (AR) in native valves1. The high spatial and temporal resolution achievable with echocardiography is unmatched and allows good visualisation of valve morphology. Precise characterisation of the magnitude of valvular regurgitations still remains one of the major challenges, especially with eccentric jets. In the clinical context, significant limitations in the different methods of quantifying valvular regurgitations have been reported2. Angiocardiography with iodine contrast media3,4 was the first quantification method that being considered over the years as a reference standard. Various echocardiographic techniques have been used to estimate the severity of valvular regurgitation5. For AR, echocardiographic indicators used to assess severity include regurgitant jet length and area6, Doppler signal strength, width of the proximal colour Doppler jet5 and deceleration slope of the continuous Doppler wave form estimated by measurement of pressure half time7.
Transcatheter aortic valve implantation (TAVI) using the Medtronic CoreValve bioprosthesis (Medtronic, Minneapolis, MN, USA) has been associated with a high rate of paravalvular prosthetic regurgitation, usually mild (echocardiographically evaluated), with an incidence ranging from 86% to 95%8,9. The presence of the severely calcified native valve between the percutaneously implanted bioprosthesis and the aortic annulus probably precludes a complete sealing of the paravalvular space, and thereby leads to some degree of AR in most cases.
Cardiac magnetic resonance (CMR) phase-velocity mapping for measurement of blood flow volumes has been validated in vitro and in vivo10. Velocity-encoded CMR allows measurement of blood flow velocity and volume as well as estimating regurgitant fraction (RF) from the ratio of forward to backward flow across the valve11. The utility of the velocity mapping technique by CMR has been validated successfully for quantification of AR12, and has been shown to be in good agreement with the angiographically obtained grade of AR13.
We hypothesised that echocardiography tends to underestimate the degree of paravalvular AR after TAVI using the Medtronic CoreValve bioprosthesis due to the eccentricity of the jets, and that CMR can accurately evaluate the severity of AR, especially in patients with unexplained symptoms of heart failure after valve implantation. Therefore, this study aims to evaluate the accuracy and feasibility of CMR in quantifying regurgitant volume (RV) and RF in patients treated with the Medtronic CoreValve bioprosthesis for severe calcific aortic stenosis, and to compare the results with echocardiography and aortography.
Patients and methods
This pilot study included 16 patients with a mean age of 78.7 years (eight women, eight men) who underwent successful TAVI using the Medtronic CoreValve bioprosthesis. AR was investigated by CMR, echocardiography, and aortography. Angiographic grading of AR was done immediately after valve implantation. CMR and echocardiography were performed four weeks after valve implantation. Patients with atrial fibrillation and severe arrhythmias or unstable conditions were excluded. All patients were informed about the potential risks of CMR and gave their written consent.
Aortography
The technique of transfemoral TAVI and valve design have been described elsewhere14. After valve implantation and pulling the wires from the ventricle, a 5Fr pigtail catheter was placed in the upper part of the implanted valve above the leaflets in the ascending aorta. Aortography in 30° RAO and 50° LAO projections (Figures 1 and 2) were recorded over several cardiac cycles. For aortography, 35ml of contrast material was injected with a flow rate of 16ml/sec. Qualitative assessment of the severity of AR was performed by visual estimation of the concentration of contrast medium in the left ventricle, using the method of Sellers et al3. Two independent interventional cardiologists have reviewed the results.
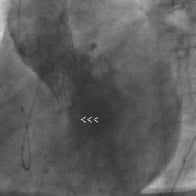
Figure 1. Ascending aorta angiography in LAO 50° showing gradeIII AR after CoreValve implantation in patient number 11 (arrowheads show eccentric regurgitant jet).
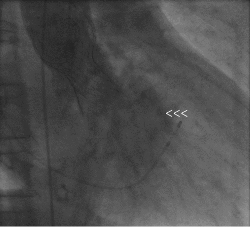
Figure 2. Ascending aorta angiography in RAO 30° showing grade III AR after CoreValve implantation (arrowheads show eccentric regurgitant jet).
Transthoracic echocardiography
All patients underwent TTE four weeks after valve implantation. AR was evaluated according to published guidelines1,15. Colour-flow techniques included measurement of the width and area of the aortic regurgitant jet at the junction of the LV outflow tract, and the aortic annulus in parasternal long axis view in relation to the maximum width and area of the LV outflow tract at the same location6. Two independent echocardiographers reviewed the results. AR was graded as I for mild, II for moderate, III for moderate to severe, and IV for severe16.
CMR
After taking into consideration the conditions under which the Medtronic CoreValve bioprosthesis can be scanned safely (mentioned in instructions for use), CMR was carried out with a 1.5-T Magnetom Espree device (Siemens AG, Erlangen, Germany), with a 33-mT·m-1 gradient and 330-µsec rise time. All patients were investigated by electrocardiogram-gated CMR in the supine position with a phased-array body coil. Initially, a turbo-fast low-angle shot sequence was obtained in various planes as localisers. A2-dimensional T2-weighted breath-hold (end-inspiration) half-Fourier acquisition single-shot turbo spin-echo sequence (HASTE; effective echo time 27ms, repetition time 750ms, slice thickness 8mm, field of view 350-400mm, multislice technique with 16slices, 106×256 matrix size) was planned on the coronal localiser for axial views. A breath-hold gradient-echo cine study in LV outflow tract was performed to visualise the direction and area of the regurgitant jet (Figure 3). For flow measurements, a breath-hold velocity-encoded phase-difference MR sequence was used (“through plane”, segmented fast low-angle shot 2-dimensional sequence, repetition time/echo time 46/2.7 ms, velocity encoding 150-300 cm·sec–1). The duration of the breath-hold period was 15-25sec. Flow measurements were performed by positioning the slice in the ascending aorta in vicinity of the upper margin of the aortic prosthesis. The total CMR examination time was approximately 30-40min for all sequences. CMR data were analysed by two independent and experienced observers who had no knowledge of the AR grades of the patients. The cross-sectional area of the aorta was defined separately on each image magnitude by a region of interest. Within these individual regions of interest, the mean flow velocity was measured on a pixel-by-pixel basis (Figures 4a and 4b). Using a work-in-progress software (Argus WIP 2.3, Siemens AG, Erlangen, Germany), the forward and reverse volumes and the net forward volume were determined, and RF was calculated (Figure 5). A calculated RF of 0%-15% was graded I (mild), 16%-30% was graded II (moderate), 31%-50% was graded III (moderate to severe) and > 50% was graded IV (severe), according to the standard grading criteria17.
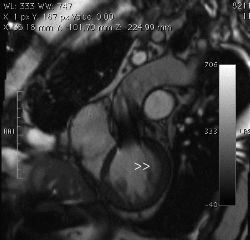
Figure 3. CMR left ventricular outflow view in diastole shows eccentric aortic regurgitant jet (arrowheads).
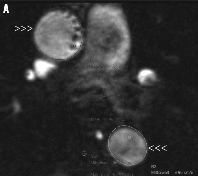
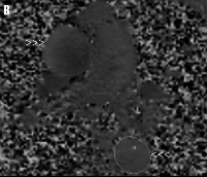
Figure 4. A) Magnitude phase-contrast MR image used for anatomic correlation. B) Velocity MR image: upper arrowheads show ascending aorta at the level of the pulmonary artery bifurcation, the lower show the descending aorta.
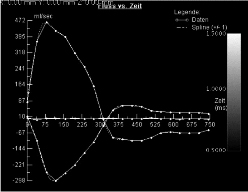
Figure 5. Profile of aortic flow rate during cardiac cycle in patient number 11 with grade III aortic regurgitation quantified with MR velocity mapping (RF=31%). Areas under the positive and negative parts of the curve represent forward and regurgitant flow, respectively. The upper curve corresponds to flow in the ascending aorta, while the lower curve corresponds to flow in the descending aorta.
Statistics
Descriptive results were expressed as mean ± standard deviation for continuous variables. To correlate the different methods, the Spearman coefficient was used. To describe the strength of agreement between measurements, weighted kappa measurement was done.
Results
The detailed characteristics of each patient and the mean AR grades obtained by the three methods are shown in Table 1. The direction of the AR jet was eccentric in all cases, and in 25% of the cases there was more than one jet. There was a highly significant correlation between CMR and angiographic degree of AR (r=0.86, p<0.001, Figure 6). On the other hand, there was a limited correlation between CMR and echocardiography regarding the degree of AR (r=0.374, p=0.15, Figure 7), the same also between echocardiography and angiography (r=0.319, p=0.23, Figure 8).
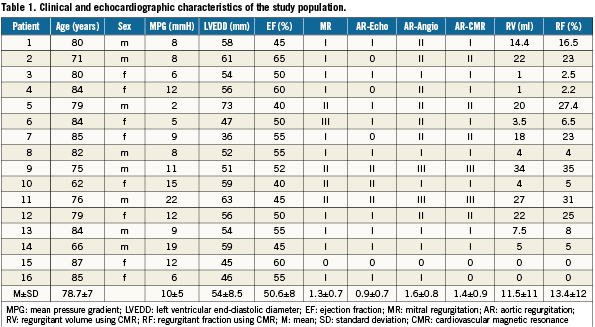
Figure 6. Scatter-plots of the correlation between angiography and CMR measurements are shown. The Spearman’s rank correlation is 0.864, with p<0.001 for the test of zero correlation.
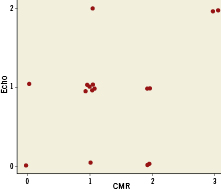
Figure 7. Scatter-plots of the correlation between echocardiography and CMR measurements. The Spearman’s rank correlation between these two measurements is 0.374, with p=0.15 for the test of zero correlation.
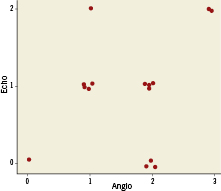
Figure 8. Scatter-plots of the correlation between echocardiography and angiography measurements are shown. The Spearman’s rank correlation between these two measurements is 0.319, with p=0.23 for the test of zero correlation.
Confirming the above results, the weighted kappa for agreement between echocardiography and angiography was 0.14, for agreement between echocardiography and CMR 0.20, and for agreement between angiography and CMR 0.72.
Echocardiography underestimated AR by one degree compared to CMR in five patients and 2 degrees in two patients; in six of these, the degree of AR obtained by CMR was similar to angiography. Noteworthy, two patients (numbers 9 and 11) had recurrent hospitalisation because of symptoms of heart failure, the degree of AR was estimated II by echocardiography, and III by CMR. The cause of AR was identified by CMR in one patient as a sharp protruding calcified spike that had created a gap between the external stent surface and the native leaflets’ internal surface (Figure 9).
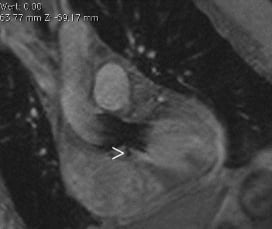
Figure 9. CMR LV outflow tract view shows a calcified spike (arrowhead) creating a gap between the external stent surface and the native leaflets.
Noteworthy, CMR showed the lowest interobserver variability, with a kappa value of 0.854 compared to 0.475 with echocardiography and 0.523 with angiography.
Discussion
The current study is the first to use CMR to assess the degree of AR after TAVI and to compare the quantitative RF and RV obtained from CMR with the echocardiographic and angiographic-derived qualitative estimates of AR.
We found that, after TAVI using the Medtronic CoreValve bioprosthesis, echocardiography may underestimate the degree of AR, and CMR in these circumstances has a great potential to reliably measure the severity of AR.
Owing to the three-dimensional nature of the aortic regurgitant jets, qualitative assessment in two dimensions can be difficult. Eccentric jets like those we see after TAVI and the mitral valve inflow signal can interfere with colour flow mapping of AR18. In some patients, after TAVI, the presence of moderate to severe AR may lead to development of symptoms of heart failure, because these patients often have small, non-compliant ventricles. Echocardiography, the standard method for diagnosing AR, may underestimate this complication in such patients. Therefore, the need for a non-invasive imaging tool to evaluate the degree of AR after TAVI is warranted.
With the introduction of velocity-encoded phase-difference CMR, an accurate and direct measurement of blood flow velocity and regurgitant volume is possible19.
Echocardiography versus CMR
Fast and easy estimation of the degree of AR with no special analysis software and the fact that it is a bed-side modality, are the main advantages of echocardiography over CMR. Disadvantages are that it allows only semi-quantitative estimation and eccentric jets are possibly underestimated. Eccentric jets may become entrained along the LV wall, which tends to alter their appearance and hence the perception of AR severity20. In addition to the regurgitant flow, a set of interrelated physical and haemodynamic variables influence the spatial dispersion of the regurgitant jets, as the pressure in the receiving chamber21. The pressure gradient through the orifice seems to be the most important factor for determining jet dimensions21. This can explain in part the inaccuracy of echocardiography in evaluating AR after TAVI, as in spite of the relieved stenosis and afterload, the occurrence of AR can contribute to elevation of the LV end-diastolic filling pressures that affects the appearance of the regurgitant jet and leads to underestimation of the degree of AR. Clinical studies22 have shown that the area of an eccentric jet that adheres to a wall may reach a magnitude equivalent to 40% of the one observed for a central regurgitant jet corresponding to the same regurgitant volume. Moreover, the configuration of the implanted prosthetic valve may have an influence on the distribution of the regurgitant jet, which may contribute to the inaccuracy in estimating the regurgitation severity. Shielding and artefacts may hinder insonation of the prosthetic valve and especially of regurgitant jets associated with the valve.
On the other hand, CMR allows better estimation of AR, even in patients with multiple valve disease, because RV determination is independent of the presence of mitral regurgitation23. Another advantage of CMR is the low interobserver variability. Consistent with our findings, Engels et al24 found better correlation with the clinical grade of valve disease in adults using CMR flow measurements rather than echocardiography.
Quantitative flow measurement with CMR is a reliable and robust technique; however, errors and inaccuracies can easily be introduced without careful attention to detail. Most potential errors are manageable and can be limited by careful attention to technique. Some errors are intrinsic to the method and anatomy and cannot be fixed by using currently available technology. Choosing the correct value for the velocity-encoding gradient is one important detail18.
CMR is unlikely to replace echocardiography as the primary modality for assessment of valvular regurgitations. The superior spatial and temporal resolution of echocardiography is a clear advantage over CMR in evaluation of valve morphology18. Nevertheless, CMR is more accurate at quantification of ancillary findings in valve disease especially in estimating regurgitant volumes. Direct measurement of flow is possible with CMR but not with echocardiography18.
Noteworthy, we have chosen the angiographic grading of AR as the standard reference, owing to the semi-quantitative nature of this grading system that correlates well with the percentage and volume of regurgitation25.
Limitations
The main limitation of our study is the small number of patients. Comparison of our findings to the literature might be inappropriate, as we have assessed the correlation between different methods in a special category of patients that was not assessed before. Moreover, all regurgitant jets assessed were eccentric, which might have an influence on the interpretation of the echocardiographic results. Detailed work about the causes of AR after TAVI using CMR should be addressed in future studies. The cost of CMR may represent an important limitation in comparison to echocardiography.
Conclusion
In patients treated with TAVI, comparisons between purely quantitative measurements of aortic RF and RV by CMR and qualitative assessment of AR by angiography showed better correlations than with echocardiography. TTE may underestimate the degree of AR in patients treated with the Medtronic CoreValve bioprosthesis, CMR may have great potential in reliably measuring the severity of AR in a quantitative manner in these patients, and may provide additional functional and anatomical information leading to a better understanding of patient outcome.
Acknowledgement
Our thanks to Dr. Derek R. Robinson (senior lecturer in statistics, University of Sussex, Brighton, UK) for his professional statistical support during this work. Likewise, our thanks to the clinical research group at the Heart and Vascular Centre, Segeberger Kliniken GmbH, especially Mrs. Daniela Schuermann-Kuchenbrandt and Mr. Guido Kassner.
Conflict of interest statement
The authors have no conflict of interest to declare.

