Abstract
Aims: Aortic regurgitation (AR) after Medtronic CoreValve System (MCS) implantation may be explained by patient-, operator- and procedure-related factors. We sought to explore if frame geometry, as a result of a specific device-host interaction, contributes to AR.
Methods and results: Using rotational angiography with dedicated motion compensation, we assessed valve frame geometry in 84 patients who underwent TAVI with the MCS. Aortic regurgitation was assessed by angiography (n=84, Sellers) and echocardiography at discharge (n=72, VARC-2). Twenty-two patients (26%) had AR grade ≥2 using contrast angiography, and 17 (24%) by echocardiography. Balloon predilatation and sizing and depth of implantation did not differ between the two groups. Despite more frequent balloon post-dilatation in patients with AR (40.9 vs. 9.7%, p=0.001), the frame was more elliptical at its nadir relative to the patient’s annulus (6±13 vs. –1±11%, p=0.046) and occurred in a larger proportion of patients (61.9 vs. 26.8%, p=0.004). Although the Agatston score and the eccentricity of the MCS frame relative to the annulus were independent determinants of AR (odds ratio: 1.635 [1.151-2.324], p=0.006, and 4.204 [1.237-14.290], p=0.021), there was a weak association between the Agatston score and the adjusted eccentricity (Spearman’s rank correlation coefficient ![]() =–0.24, p=0.046).
=–0.24, p=0.046).
Conclusions: These findings indicate that AR can be explained by a specific device-host interaction which can only partially be explained by the calcium load of the aortic root.
Abbreviations
AR: aortic regurgitation
ESV: Edwards SAPIEN valve
MSCT: multislice computed tomography
MCS: Medtronic CoreValve System
R-angio: rotational angiography
TAVI: transcatheter aortic valve implantation
Introduction
Transcatheter aortic valve implantation (TAVI) is increasingly being used to treat patients with aortic stenosis who are considered too high a risk for surgical valve replacement1-5. A vexing clinical problem is the occurrence of paravalvular aortic regurgitation (AR) which is reported to occur more frequently after implantation of the self-expanding Medtronic CoreValve® (MCS) (Medtronic, Minneapolis, MN, USA) than after the balloon-expandable Edwards SAPIEN valve (ESV) (Edwards Lifesciences, Irvine, CA, USA)6-8.
Patient and procedure-related variables such as aortic root calcification, annular dimensions, depth of implantation and sizing, have been identified as determinants of AR post TAVI9,10. However, specific valve-related issues such as the type (i.e., circular vs. non-circular expansion) and degree of valve expansion may play a role as well. There is evidence from multislice computed tomography (MSCT) analysis in selected patients that non-circular expansion and malapposition is more frequent after MCS than ESV valve implantation11-14. The question is to what extent this plays a role in the development of AR on top of patient-related and procedural variables. For that purpose, we have adopted a strategy of performing routine rotational angiography (R-angio) using dedicated motion compensation to study frame geometry immediately after valve implantation15. The purpose of this study was to assess the correlation between MCS geometric findings by rotational angiography and AR after TAVI.
Methods
PATIENTS
The study population consisted of 98 consecutive patients who underwent TAVI with the MCS valve in whom R-angio with motion compensation was performed15. Only patients with sufficient image quality for frame assessment (grade 1,2,3) were included using the following scores:
Grade 1: excellent image quality (struts visible without artefacts).
Grade 2: struts clearly visible, distinction between struts and artefacts possible.
Grade 3: struts visible but in some regions distinction between struts and artefacts cannot be made.
Grade 4: degraded (struts are blurred and distorted).
Grade 5: strongly degraded (struts and artefacts cannot be distinguished) (Figure 1).

Figure 1. Image quality of R-angio: grade 1 (best quality) to grade 5 (worst quality). CT: central coaptation of the leaflets; I: inflow; N: nadir
A total of 14 patients were excluded from the analysis because of image quality grade 4 (six patients) and grade 5 (eight patients). Therefore, the final study population was 84.
Patients were seen at the outpatient clinic and gave written informed consent for anonymised prospective data collection for clinical research purposes (TAVI Care & Cure project, MEC-2014-277). All patients underwent TAVI under general anaesthesia via the transfemoral approach, except two in whom the subclavian approach was used following Heart Team discussion. MSCT was used for sizing in all except nine patients16.
ROTATIONAL ANGIOGRAPHY, 3D RECONSTRUCTION AND FRAME ANALYSIS
R-angio was performed immediately after TAVI using the Artis zee biplane angiographic C-arm system (Siemens AG Healthcare, Forchheim, Germany). The technical details have been described before15. Cross-sectional short-axis images were used for frame analysis (Figure 2).
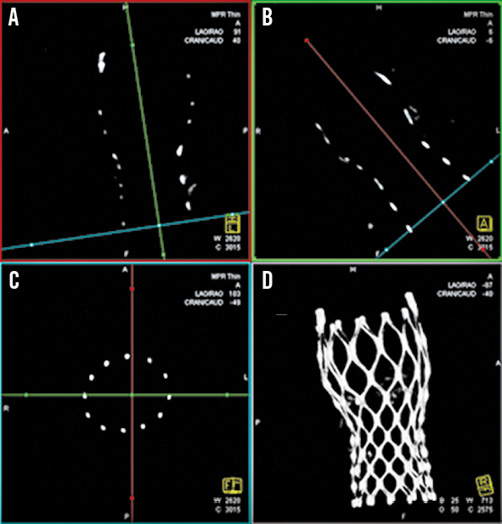
Figure 2. Acquisition of the multiplanar reformatted short-axis view (C) at the different levels of interest adjusting two longitudinal multiplaner reformatted orthogonal views (A & B) similar to MSCT16 and the resulting volume-rendered tridimensional reconstruction (D).
Frame analysis: MCS frame analysis was performed at three predefined levels (Figure 3), inflow, nadir and central coaptation of the leaflets13. At each of these levels, the minimum diameter (Dmin), maximum diameter (Dmax), area and perimeter were manually measured using the centre point of the strut(s).
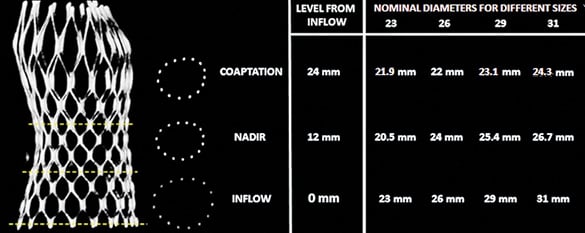
Figure 3. Cross-sectional view at the three levels of interest of the MCS frame. Nominal perimeters at the various levels of the MCS frame were kindly provided by Medtronic Inc., Minneapolis, MN, USA.
Expansion of the frame was calculated by measured perimeter/nominal perimeter. The eccentricity of the frame was calculated by Dmin/Dmax×100. The eccentricity of the frame at the level of the nadir was adjusted to the eccentricity of the native annulus using the following equation: (Eccentricity nadir – Eccentricity native annulus/Eccentricity native annulus)×100, since this part of the frame, that contains the nadir of the bioprosthetic leaflets, is in closest contact with the base of the aortic root and is the part most subjected to the constraining forces of the aortic root.
ASSESSMENT OF AORTIC REGURGITATION
Contrast angiography and Doppler echocardiography were used to assess AR immediately after TAVI and at discharge17,18. With respect to contrast angiography, AR severity was defined using the Sellers classification (0=none, 1=mild, 2=moderate, 3=moderate to severe, and 4=severe)17. For that purpose, an angiography protocol was used consisting of the injection of 20 ml of non-diluted Iodixanol (Visipaque™; General Electric Company, Fairfield, CT, USA) at a flow rate of 20 ml/sec via a 6 Fr pigtail catheter which was positioned above the bioprosthetic leaflets. Cine runs were recorded at a speed of 30 frames/sec. Two observers (independently from one another) scored the angiograms. In case of discrepancy, consensus was reached by including a third observer. The intra- and inter-observer variability for the assessment of AR post TAVR according to the Sellers classification was κ 0.70, 0.60 and 0.78, respectively. A distinction was made between patients with Sellers grade 0-1 and those with Sellers grade 2-4.
Doppler echocardiography was performed before discharge. AR severity was defined by the circumferential extent of the Doppler signal at the inflow of the MCS frame in the parasternal short-axis view (SAX) using the VARC-2 criteria18. Echocardiography was available in 72 out of the 84 patients. A distinction was made between none-mild (<10%) and moderate-severe (10-29% and ≥30%) AR.
STATISTICS AND ANALYSIS
Categorical variables are presented as frequencies and percentages and compared using Pearson’s chi-square test. Continuous variables are presented as means (±SD) and compared with the Student’s t-test. The association between two continuous variables was carried out by using Pearson’s or Spearman’s rank correlation coefficient test when adequate. To study the independent predictors of AR post TAVI, logistic regression was performed. All characteristics judged to be clinically relevant or to have a pathophysiologic role in AR post TAVI were included in the multivariable logistic regression model. A two-sided alpha level of 0.05 was used to indicate significance. Statistical analyses were performed using SPSS software, Version 21.0 (IBM Corp. Armonk, NY, USA).
The main analysis consisted of the detection of the determinants of AR based on comparing patients with Sellers grade 0-1 and 2-4 on angiography after implantation. The secondary analysis consisted of the assessment of the determinants of AR by comparing patients with no or mild (<10%) and those with moderate or severe AR (10-29% and ≥30%) on the SAX view of the echo-Doppler examination before discharge18-20.
Results
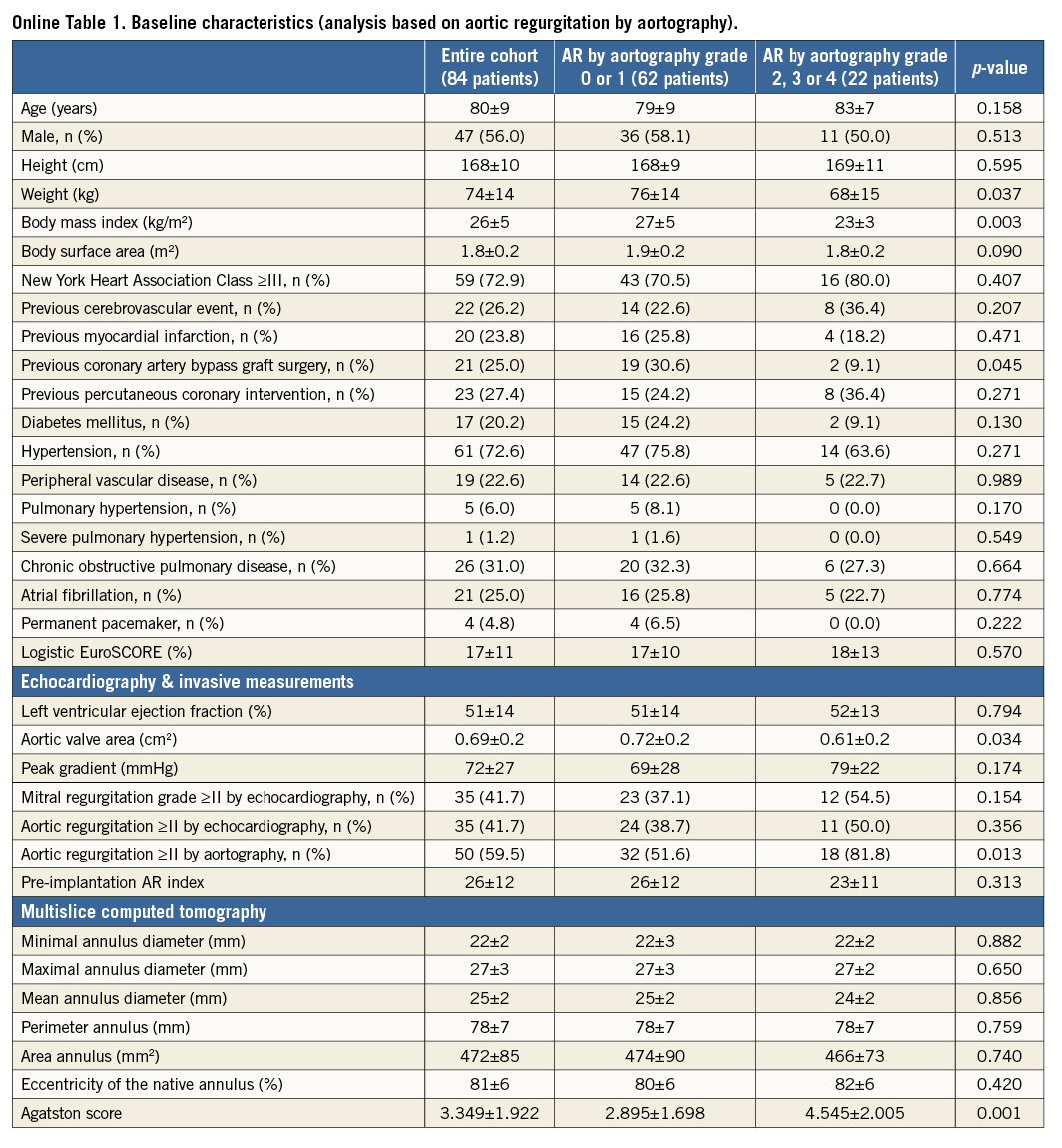
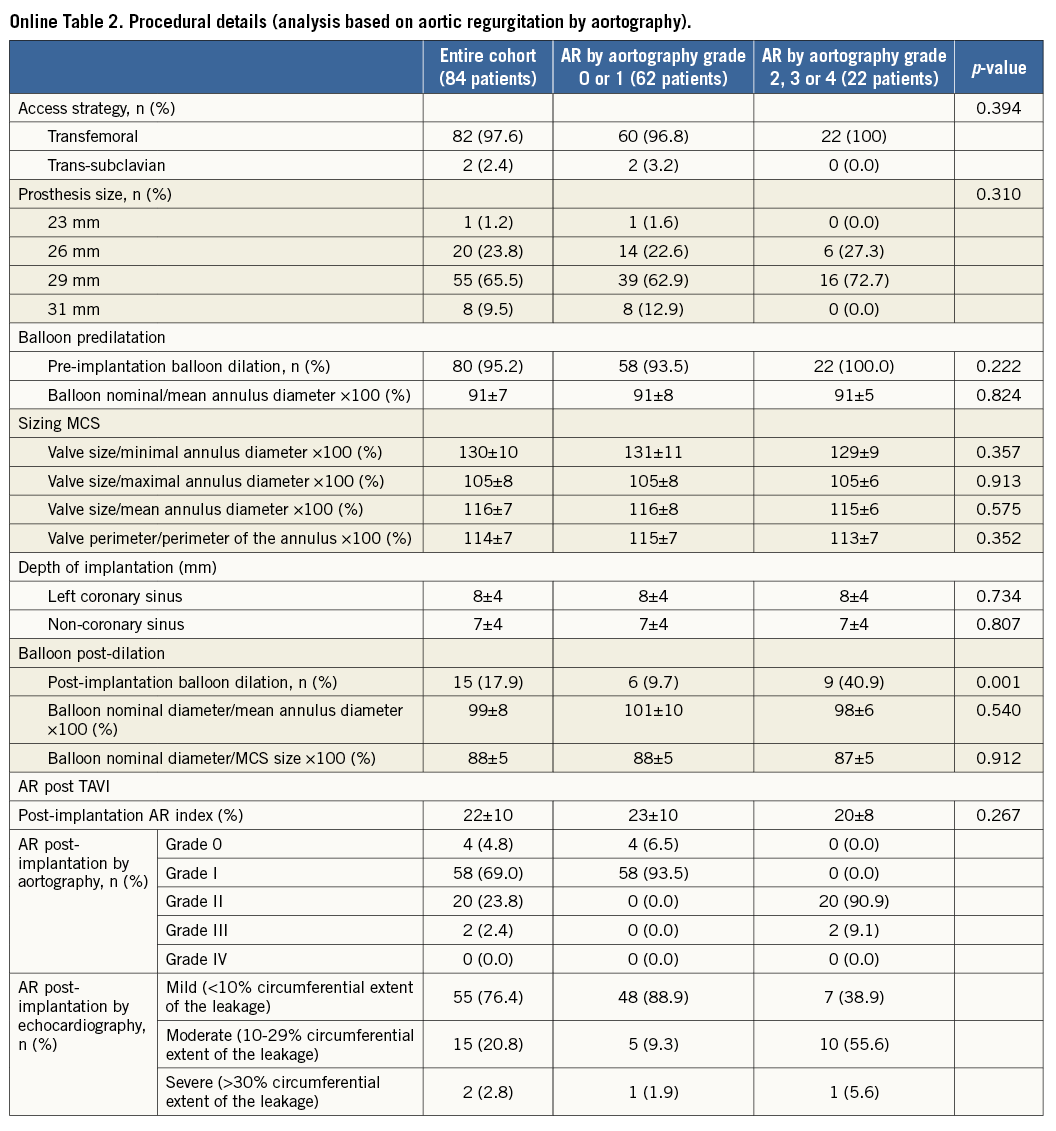
The baseline clinical and procedural data of all patients and of those with AR grade 0-1 versus those with AR grade ≥2 post TAVI are summarised in Online Table 1 and Online Table 2.
AR grade ≥2 was seen in 22 patients (26%). By univariable analysis, these patients had a lower body weight (68±15 vs. 76±14 kg, p=0.037) and body mass index (23±3 vs. 27±5 kg/m2, p=0.003), and a lower prevalence of antecedent coronary artery bypass surgery (9.1% vs. 30.6%, p=0.045). They also had more severe aortic stenosis (aortic valve area of 0.61±0.2 cm2 vs. 0.72±0.2 cm2 [p=0.034]) with a higher prevalence of AR at baseline by aortography (AR ≥2 81.8% vs. 51.6% [p=0.013]) and a higher Agatston score (4.545±2.005 vs. 2.895±1.698, p=0.001).
From a procedural perspective, there was no difference in predilation strategy, sizing and depth of implantation between the two groups. The only difference was a higher frequency of balloon post-dilatation in patients with AR grade ≥2 (40.9% vs. 9.7%, p=0.001).
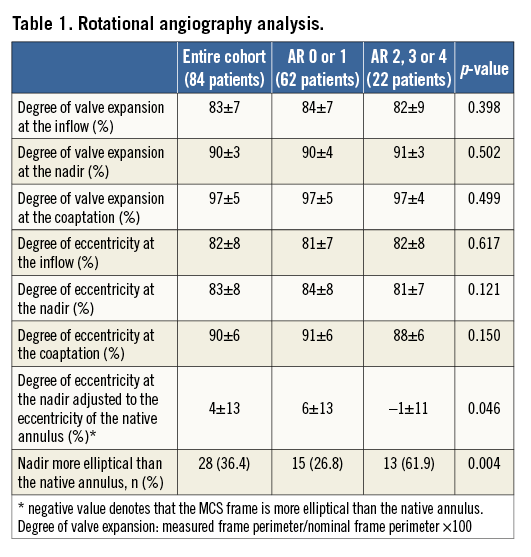
With respect to frame geometry (Table 1), there was no difference in the degree of frame expansion or eccentricity at any level between patients with AR grade 0-1 and ≥2. Yet, when relating the degree of frame eccentricity at the level of the nadir with the degree of eccentricity of the native annulus, the frame was more elliptical at its nadir relative to the patient’s annulus in patients with AR ≥2 compared to those with AR grade 0-1 (6±13 vs. –1±11%, p=0.046).
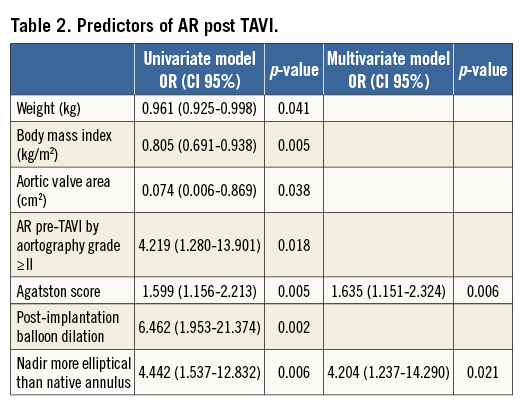
The eccentricity of the frame relative to the patient’s annulus was also seen in a larger proportion of patients with AR ≥2 (61.9 vs. 26.8%, p=0.004). In other words, when the valve was more elliptical than the recipient’s anatomy (adjusted ellipticity <0) there was significantly more AR than when the valve was less elliptical than the annulus (adjusted ellipticity ≥0).
Spearman’s rank correlation coefficient revealed a weak association between Agatston score and the adjusted eccentricity (=-0.24, p=0.046). There was no correlation between prosthesis sizing and adjusted eccentricity (R=0.03, p=0.81).
Multivariate analysis revealed the Agatston score and eccentricity of MCS frame relative to the native annulus to be independent determinants for AR post TAVI (odds ratio: 1.635 [1.151-2.324], p=0.006, and 4.204 [1.237-14.290], p=0.021, respectively) (Table 2).
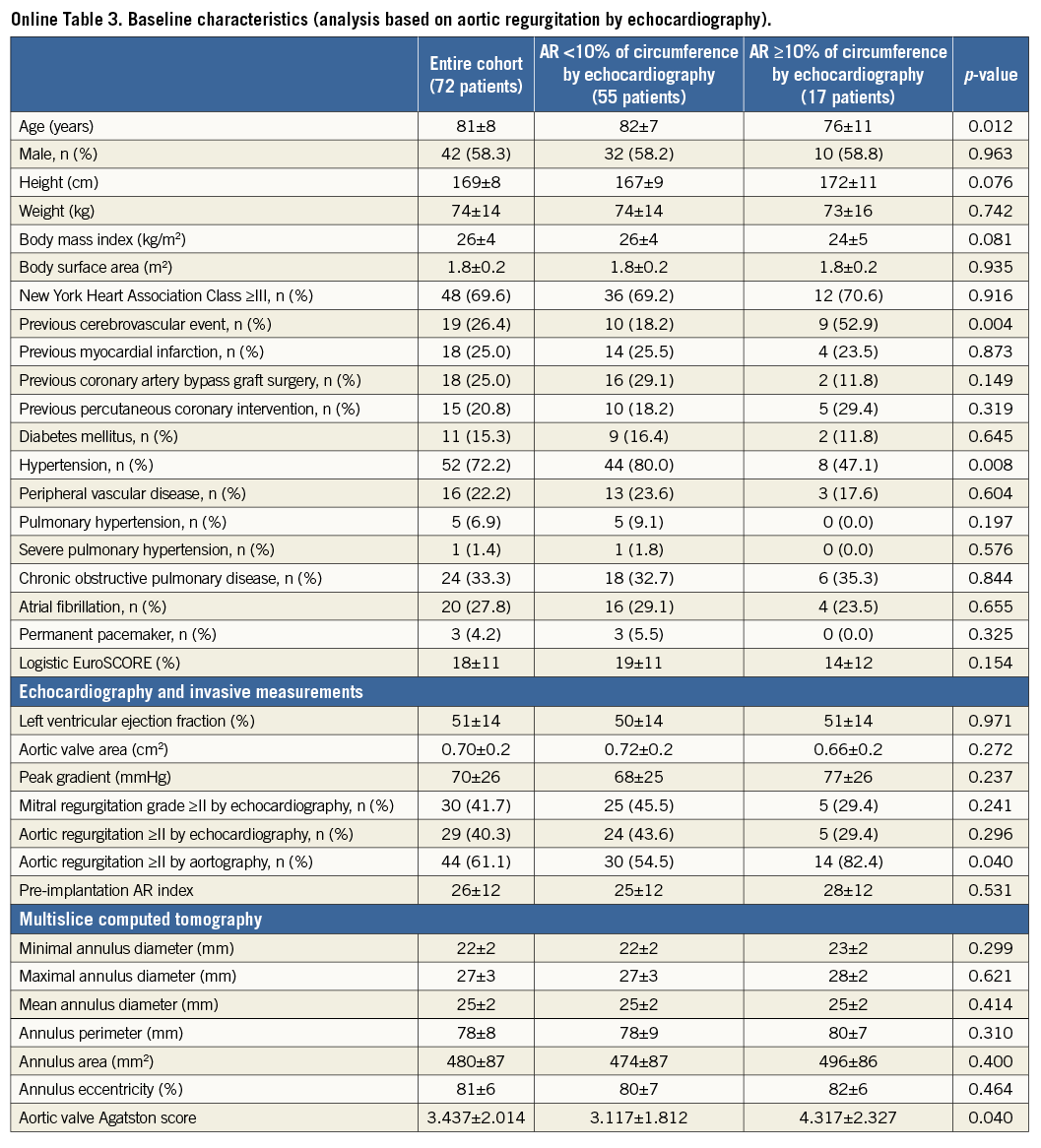
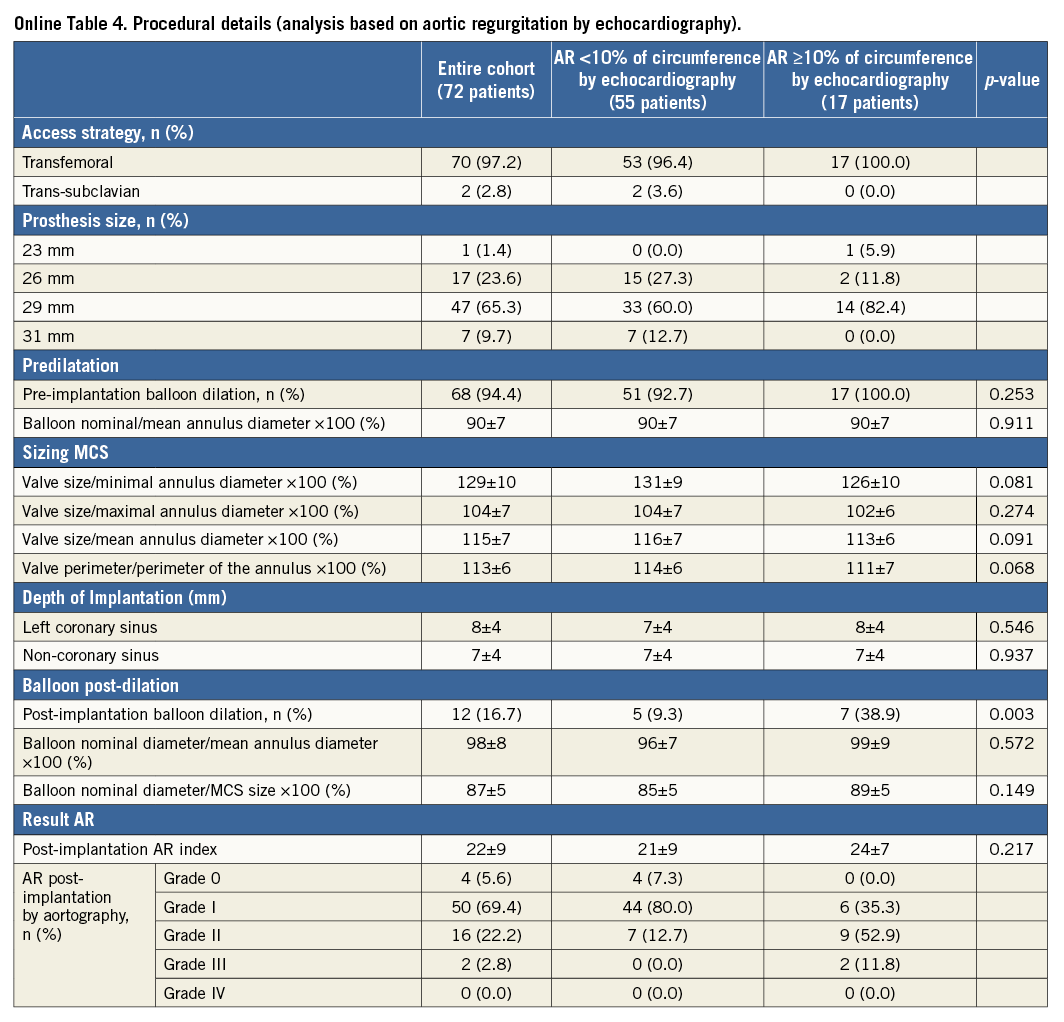
Repeat analysis based on the circumferential extent of AR on echocardiography before discharge indicated similar findings but lacked statistical power to confirm (Online Table 3-Online Table 5).
Discussion
The main finding of this study is that, in patients with severe aortic stenosis who received the self-expanding MCS valve, the frame was more elliptical at its nadir relative to the patient’s annulus in patients with >mild AR (6±13%) compared to those with no or mild AR (-1±11%). The eccentricity of the frame relative to the patient’s annulus was also seen in a significantly larger proportion of patients (61.9 vs. 26.8%, p=0.004) despite a more frequent use of balloon dilatation after valve implantation (40.9 vs. 9.7%, p=0.001). Although the Agatston score and degree of adjusted ellipticity were independent determinants of AR, the Spearman rank correlation analysis indicates that the calcium load of the aortic root only partially explains the degree of adjusted ellipticity.
These data indicate the presence of a specific device-host interaction that, in turn, may suggest the presence of incomplete apposition. Unfortunately, apposition, or the lack thereof, cannot be assessed in vivo. This is supported by experimental findings analysing the numerical radial force of the MCS valve that was used in the current population21. Tzamtzis et al found that the radial force of the MCS frame rapidly drops with the increasing diameter of the recipient’s anatomy and, depending on this diameter, may reach zero21. The model did not include elastic coupling between valve and host tissue and may therefore underestimate the radial force exerted by the frame in vivo but supports the findings in this clinical study and, therefore, the aetiology of AR. Furthermore, the experimental model of Tzamtzis used a cylindrical surrogate for the annulus while in patients it is often asymmetric11-14. Therefore, non-uniform distribution of force along the perimeter of the MCS valve most likely occurs, which, in combination with opposing forces due to calcium, may affect apposition in vivo.
Lack of apposition as a cause of AR after MCS, on top of other factors such as sizing and depth of implantation, is supported by the findings in 56 patients treated with the MCS valve in whom geometric analysis of the MCS frame (cardiac CT post TAVI) was correlated with AR on the short-axis view of transthoracic echo-Doppler cardiography (SAX-TTE)22. Malposition was reported in 35 patients (63%) and was correlated with the calcium load of the aortic root and the site of AR on SAX-TTE22. The role of apposition as a cause of AR and the effects of calcium on apposition are highlighted by the findings in another 110 patients treated with the MCS valve in whom it was found that aortic root calcification had a higher discriminatory power for the prediction of balloon dilatation after MCS valve implantation to treat AR than sizing (prosthesis to annulus ratio)23.
Tzamtzis et al also demonstrated a similar biomechanical behaviour in the Edwards SAPIEN valve (stainless steel frame - decreasing radial force with increasing diameter of the recipient anatomy) but found that it was independent of tissue stiffness21. This may explain why (at variance with the MCS valve) circularity after ESV implantation is the rule: circularity of the ESV valve was reported in all but two out of 89 patients (98%) and was independent of the native annular anatomy while symmetrical expansion of the MCS frame was seen in only five out of 30 patients (17%)13,14. The aggregate of these data indicates that the MCS conforms to the geometry of the patient’s annulus while the ESV dictates the geometry of the annulus. Interestingly, in this study patients with AR had a more severe aortic stenosis (AVA) and a higher Agatston score, reflecting more advanced atherosclerotic disease and therefore higher opposing forces to frame expansion. The combination of the experimental findings of Tzamtzis et al and the current clinical findings indicate that some extra degree of oversizing may be needed when using the MCS valve, in particular in such patients, to overcome the calcium load (in addition to correct positioning) or to change the biomechanical properties of the MCS frame at the inflow level. Unfortunately, this study lacks the power to prove this point. Another solution is the use of a repositionable valve which allows the operator to change the depth of implantation so as to avoid or minimise AR24,25.
In comparison with the CHOICE study which compared valve function between the MCS and ESV, contrast angiography was the principal method used to address the current study objective8. AR is most often caused by inappropriate sealing or apposition of the valve, though sometimes it is due to a central leak. This distinction cannot be made by angiography and clarification is needed for proper definition of the eventual additional treatment, such as balloon dilatation in case of malapposition. With respect to quantitative assessment of AR, the limitations of echocardiography are well known19,20. MRI has been shown to be the best method but is less available in clinical practice26,27.
In this study, R-angio was used for the assessment of frame geometry using dedicated prototype software for motion compensation which was validated with MSCT15. The advantage of R-angio over MSCT is that it is available on-line in the catheterisation or hybrid operating room. The morphologic information of the valve in combination with haemodynamic (e.g., residual gradient) and/or echocardiographic findings during TAVI may help to tailor or define additional therapeutic measures to improve outcome and valve function28.
Limitations
The main limitations of the present study are sample size and the absence of independent (core lab) analysis of the parameter of interest. Also, not all patients underwent echocardiography before discharge and we did not perform rotational angiography before and after additional balloon dilatation. A larger sample would have allowed a more comprehensive and robust multivariable analysis. In addition, a larger or multicentric sample with a more heterogeneous distribution of the independent variables –sizing and depth of implantation in particular– would have allowed the assessment of the strength of the reported specific device-host interaction relative to operator- and procedure-related variables such as sizing and depth of implantation. The present analysis also pertains to a selected group of patients (i.e., patients with rotational angiography with sufficient image quality). The absence of independent analysis may affect the validity of the reported point estimate of the target parameter and may explain the difference in AR between this and other studies using contrast angiography. However, we believe that the proposed aetiology of AR in the present population is plausible, in particular considering the experimental analysis of the biomechanical properties of the frame and previous clinical observations.
Conclusions
When the valve is properly implanted, AR post MCS valve implantation is the result of a specific device-host interaction (inadequate frame apposition due to calcium). It is currently unknown how to incorporate the calcium load of the aortic root into the sizing matrix.
| Impact on daily practice When the valve is properly implanted, AR post MCS valve implantation is the result of a specific device-host interaction (inadequate frame apposition due to calcium). The impact of these findings are: 1) clinically, the importance of oversizing when using the MCS valve and the development of an algorithm including calcium of the aortic root in the sizing matrix, and 2) from an R&D perspective, the development of a frame with enhanced hoop force at inflow. |
Guest Editor
This paper was guest edited by Alec Vahanian, MD, PhD; Département de Cardiologie, Hôpital Xavier Bichat, Faculté Paris Diderot, DHU FIRE, Paris, France.
Conflict of interest statement
Guenter Lauritsch is an employee of Siemens AG. The other authors have no conflicts of interest to declare. The Guest Editor has carried out low-level consultancy work for Medtronic.
Disclaimer
The concepts and information presented in this paper are based on research and are not commercially available. The product names and/or brands referred to are the property of their respective trademark holders.
Supplementary data