Abstracts
Aims: The clinical value of optimising implant angles during transcatheter aortic valve replacements (TAVR) remains undefined. The Aortic Valve Guide (AVG) is a proprietary software that provides structured analysis of three-dimensional images from rotational angiography (DynaCT). This study compares AVG with preprocedural multislice computed tomography (MSCT) and DynaCT in optimal implant angle prediction for TAVR, and evaluates if an optimised implant angle is associated with reduced paravalvular regurgitation (PVR).
Methods and results: One hundred and six consecutive patients were included, comprising three groups. Group 1 (n=19) underwent no preprocedural MSCT or DynaCT (or AVG); Group 2 (n=44) underwent periprocedural DynaCT, without AVG; Group 3 (n=43) had DynaCT with AVG. Implant angles yielded were graded as excellent, satisfactory or poor. Group 3 were more likely than Groups 2 and 1 to have excellent implant angles (83.7% vs. 52.3% vs. 42.1%, respectively, p=0.001). In 100 patients who had 30-day transthoracic echocardiogram follow-up, an excellent implant angle was significantly more likely to be associated with no PVR than a non-excellent angle (41.3% vs. 21.6%, respectively, p=0.045), independent of operator experience and THV used.
Conclusions: Optimising implant angles may be important in reducing PVR. This is significantly more likely to be achieved with AVG rotational angiography.
Introduction
Transcatheter aortic valve replacements (TAVR) have been performed in more than 40,000 patients worldwide. Transcatheter heart valves (THV) have been shown in randomised controlled trials to improve hospitalisation rates and mortality in patients deemed inoperable1. The importance of preprocedural multislice computed tomography (MSCT) is well established in patient selection2-5, valve size selection6,7, and implant guidance8,9. The quality of intraprocedural fluoroscopic guidance is crucial in the optimal placement of the THV along the aorto-ventricular axis. This is paramount in preventing complications from too high or too low a placement, e.g., valve embolisation, coronary ostia obstruction, acute paravalvular aortic regurgitation and conduction abnormalities. A low CoreValve® (Medtronic Inc., Minneapolis, MN, USA) THV implant was more than three times more likely to cause at least moderate paravalvular aortic regurgitation10, resulting in three times the mortality at 12 months.
The advent of three-dimensional (3-D) rotational angiography, such as DynaCT (Siemens Cardiac DynaCT –Artis zee; Siemens AG, Erlangen, Germany), has revolutionised TAVRs11. DynaCT allows infinite 3-D manipulation of the aorta until an “ideal” angle is achieved, with three aortic cusps aligned perfectly on a straight line, suggesting the aortic root is aligned perfectly perpendicular to the imaging beam. Recent data12 confirmed the feasibility and accuracy of DynaCT in Edwards SAPIEN THVs (Edwards Lifesciences, Irvine, CA, USA).
The Aortic Valve Guide (AVG) is a proprietary add-on software to DynaCT (Siemens AG, Erlangen, Germany) which automatically identifies the three cusps of the aortic valve and generates an ideal implant angle, at which all three cusps are seen and at equal distance to each other, i.e., a perfect line of perpendicularity. This obviates the need for operator manipulation as would be necessary in DynaCT without AVG.
Paravalvular regurgitation (PVR) is now increasingly recognised as an important contributor to mortality13,14. Recent data suggested even mild PVR carries a mortality penalty1. Several key factors contribute to PVR, such as undersizing of THV6,15,16, severe calcification of leaflets precluding full apposition of THV leaflets17-20, as well as too low a THV implant. Optimisation of THV sizing with MSCT had already been shown to improve outcomes6,15,16.
Previous research had established that rotational angiography with a pre-commercial version of AVG improved line of perpendicularity prediction12. However, it is unclear if this improved accuracy is clinically relevant and translates to reduced PVR. There is also currently no data on the use of AVG in CoreValve THVs. This study aims to address two issues: the accuracy of AVG over MSCT and DynaCT in the optimal implant angle prediction in both Edwards and CoreValve THVs, and the impact of optimised implant angle on PVR.
Methods
STUDY POPULATION
Consecutive patients were identified in our prospectively collected SOURCE ANZ (www.anzctr.org.au ACTRN12611001026910) database of patients having undergone TAVR from August 2008 to February 2012, at The Prince Charles Hospital, Brisbane, Australia. Both CoreValve (26 mm and 29 mm CoreValves) and Edwards Sapien THVs (23 mm and 26 mm Edwards SAPIEN and SAPIEN XT) were included. The choice of THV was at the discretion of the operators if the aortic annulus was deemed suitable for both; however, a CoreValve THV would be used if the annulus was ≥25 mm. The THV size was determined by at least two experienced echocardiologists, based on aortic annular measurements made on two-dimensional transthoracic or transoesophageal echocardiography. Determination of aortic annular size was not made routinely on MSCT during this study period. Rapid ventricular pacing during deployment of the THV was utilised for Edwards but not CoreValve THVs.
One hundred and nine patients were included in this study and in a post hoc analysis assigned to three groups based on the three strategies of implant angle generation: Group 1 (n=19) –patients who had no preprocedural MSCT and intraprocedural DynaCT; Group 2 (n=44) –patients who had intraprocedural Dyna CT (who may or may not have had preprocedural MSCT) but not AVG; and Group 3 (n=43) –patients who had DynaCT and AVG (29 of these patients also had preprocedural MSCT). Patients were excluded if a THV was implanted within a previous THV, or they received a THV within a failed prosthetic heart valve.
IMPLANT ANGLE GENERATION
The three patient groups differed in the manner in which the final implant angle was generated (Figure 1). In Group 1, multiple aortograms at finely adjusted angles were performed until an angle was deemed acceptable, with no procedural MSCT guidance. In Group 2, a DynaCT was performed on-table and the volume-rendered reconstructed aorta manually rotated until the three cusps were sufficiently aligned to the operators’ satisfaction (Online supplementary Figure 1 and Moving image 1). A check aortogram (at a volume of 25 ml) at this angle would then be performed, and further aortograms performed at adjusted angles at the operators’ discretion. This final, agreed-upon angle would then be adopted as the final implant angle. In Group 3, the patient would undergo DynaCT on-table and have AVG predict an implant angle. Again a check aortogram would be performed. If satisfactory, this would translate to being the final implant angle (Online supplementary Figure 2 and Moving image 2).
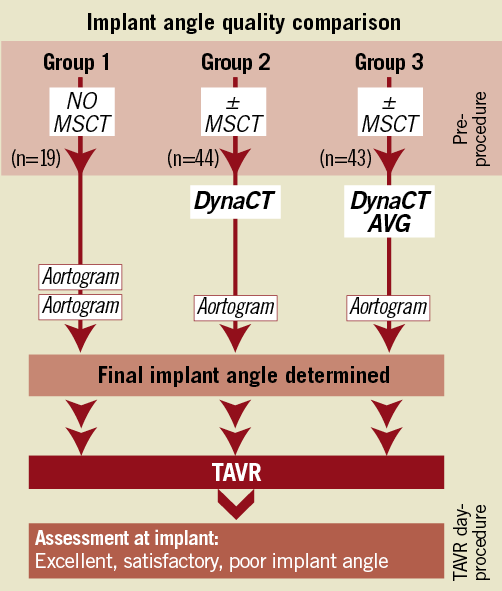
Figure 1. Study methodology – implant angle quality among the three groups.
COMPARISON BETWEEN MSCT AND AVG
The accuracy of the MSCT compared to DynaCT was specifically assessed in 29 consecutive patients in Group 3 in a novel manner different to previous published series. Each patient’s dataset from his or her preprocedural MSCT, and the dataset from the DynaCT on the implant day, were entered into the AVG workstation. The AVG software would then automatically generate an angle most coaxial for each dataset, bypassing operator interpretation. The difference between the MSCT and DynaCT angles was assessed. The respective 3-D images created were manipulated to align the sinuses at LAO 45, perpendicular and RAO 45. The corresponding caudal/cranial angulation required at these points was plotted on the chart and this demonstrated a predicted valve implantation plane from LAO 45 through to RAO 45, i.e., a line of perpendicularity.
IMPLANT ANGLE QUALITY CLASSIFICATION
Categorical evaluation of the implanted THV was determined by two blinded independent radiographers (J.C. and D.C.), based on previously utilised classification of excellent, satisfactory and poor8. In both valve types, assessment was made during diastole. There were three grades of implant angle quality: “excellent” was determined to be from perfectly aligned inferior struts, where the anterior inferior strut was up to half the height of a cell different from the posterior inferior strut; “satisfactory” was where the gap between inferior struts was determined to be from half the height of a cell to a whole cell different; “poor” implant angle was determined to be where the inferior struts were more than a whole cell different. Examples are demonstrated in Online supplementary Figure 3.
PVR ASSESSMENT
Thirty-day prospectively collected transthoracic echocardiographic (TTE) follow-up data were assessed. One hundred TTEs were available from the 106 patients for analysis (Figure 2). All TTEs were reported by experienced echocardiologists at a tertiary teaching centre, blinded to the implant angle prediction strategy utilised in each patient. PVR was categorically graded as none, mild, moderate, or severe as per VARC (Valve Academic Research Consortium) definitions.
STUDY METHODOLOGY
Correlations were made between implant angle strategies, implant angle quality and PVR severity. Baseline characteristics and procedural data were compared among the three groups. Implant angles were compared between each strategy (Figure 1). To establish the clinical impact of an implant angle, implant angle quality was correlated with the degree of PVR (Figure 2).
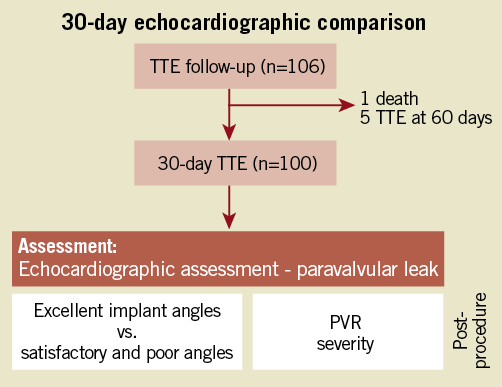
Figure 2. Study methodology – impact of implant angle quality on 30-day PVR.
The methodology of image acquisition with DynaCT and MSCT, and a brief description of AVG, are as follows:
DYNACT IMAGE ACQUISITION
Three-dimensional angiographic reconstructions were obtained with DynaCT (Siemens AG, Erlangen, Germany) rotational aortic root angiography. A 6 Fr pigtail catheter was positioned in the aortic root. A 75 ml diluted contrast medium (25 ml contrast [Ultravist 370 mg/ml], 50 ml saline) was injected and the DynaCT was acquired with rapid ventricular pacing at 180-200 beats per minute. The patient would be asked to hold his/her breath or, if intubated, ventilation would be withheld further to minimise artefact. Using the 3-D cardiac DynaCT package, the C-arm was rotated through a 200° arc in five seconds, generating 248 images. The raw data was then transferred onto a Siemens Syngo Leonardo workstation (Siemens AG) for manipulation on the InSpace 3-D package. A manual process of assigning a “cardiac smooth” modality preset and isolating the ascending aorta was performed. The reconstruction was appropriately windowed and manually rotated until the three cusps were aligned. This angle was then considered the optimised DynaCT angle.
AORTIC VALVE GUIDE
AVG (Siemens AG) is add-on software to the DynaCT system. Once the Dyna CT has been performed as above, with AVG, the dataset is automatically incorporated into the same Siemens Syngo Leonardo workstation (Siemens AG) for manipulation on the InSpace 3-D package. The software carries out a process of bone and soft tissue removal. It automatically registers the coronary artery ostia and the most inferior points of the aortic valvular sinuses, and aligns the sinuses in a linear position, all three cusps at equal distance to each other. Theoretically three such angles can be calculated, and the angle closest to the AP position is generated. The integration of the Siemens Syngo and the Artis zee allows the C-arm to be driven automatically to the displayed angle at the press of a button. A test aortogram can then be performed in the indicated position to confirm the angle is fluoroscopically satisfactory.
MSCT IMAGE ACQUISITION
A 64-slice Siemens Somatom Definition dual source CT scanner (Siemens AG) was used for all MSCTs. Scans were performed using 50 to 100 ml of intravenous contrast media (Iomeron® 350 mg [Bracco Imaging Scandinavia AB, Hisings-Backa, Sweden]). Scanner settings were 100-120 Kv, 280 mAs, rotation time 0.33 seconds, 0.27 mm pitch, slice thickness 0.6 and a 0.75 mm slice reconstruction on a b26f kernel. The images were then loaded onto the same Siemens Syngo Leonardo workstation using the Siemens Syngo InSpace software, and the AVG software was utilised.
STATISTICAL ANALYSIS
Categorical variables are presented as percentages, comparison of which was performed using a chi-square analysis. Continuous variables if normally distributed were analysed as mean±standard deviation and comparisons were made between two groups using t-test or among more than two groups using ANOVA; if not normally distributed, medians with interquartile ranges were used and a median test was performed. ORs were calculated using a logistic regression model. All analyses were performed using STATA/SE 11.2 for Windows (StataCorp LP, College Station, TX, USA).
Results
There was no difference in the baseline clinical characteristics and baseline valvular indices among the three groups.
The three groups differed in the type of THV implanted and the access utilised (Table 2). Patients in Group 1 were more likely to receive a CoreValve THV, although this reflected the earlier availability of this technology at our centre compared to Edwards SAPIEN THV. Whilst non-femoral access use differed significantly as a function of the significantly higher proportion of Edwards SAPIEN THV in Groups 2 and 3, the use of femoral access did not differ significantly among the three groups. The three groups also differed significantly in chronology, i.e., operator experience.
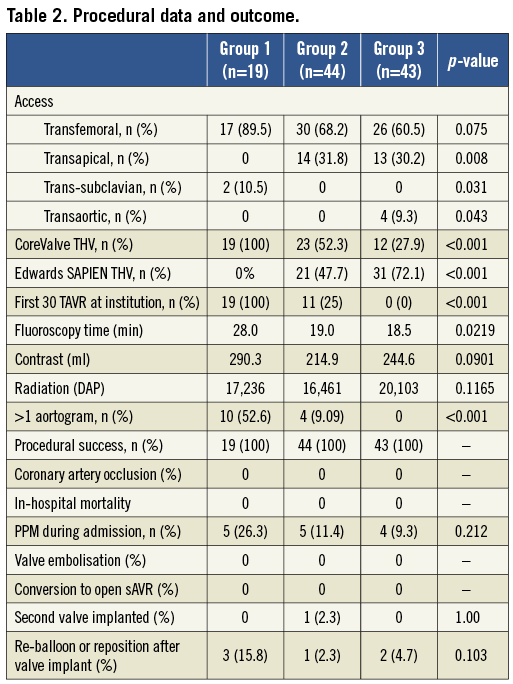
There was no adverse event after MSCT or DynaCT, such as acute renal failure requiring dialysis. No patient developed acute decompensation during rapid ventricular pacing for DynaCT requiring emergency aortic valvuloplasty. Group 3 required significantly fewer aortograms to achieve a satisfactory implant angle, although there was a trend towards higher radiation, and lower contrast use, in Group 3 compared to Group 1 and Group 2 (Table 2).
One hundred per cent procedural success, defined as the secure placement of the THV, was achieved. There was no valve embolisation, coronary ostium occlusion (Table 2). Six patients required re-ballooning (Edwards THV) or repositioning (CoreValve THV).
The mean implant angle for the entire cohort was 6.7±13.2° LAO and 4.6±9.6° caudal. In Group 3, the predicted angle from AVG was adopted by the operators in 100% of cases as the implant angle; in Group 2, 93.2% of predicted angles from DynaCT operator manipulation were adopted as the final implant angle.
Despite using an identical algorithm (AVG) to analyse datasets from MSCT and DynaCT in 29 consecutive patients in Group 3, it was demonstrated that MSCT was accurate within five degrees of the AVG predicted angle in 24.1% (7/29). At the perpendicular angle, there was a mean difference between the MSCT and DynaCT of 7.38° (±16.83°) (Figure 3).
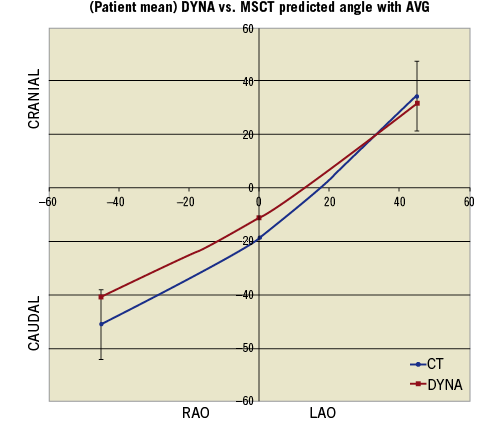
Figure 3. Comparison of angle predictions – line of perpendicularity from MSCT and AVG. The angles from each methodology at 45° RAO, 0° and 45°LAO, from the 29 patients in Group 3, were plotted.
Regarding the accuracy in predicting a co-planar angle, the use of AVG was significantly more likely to be associated with an excellent implant angle (Figure 4) over DynaCT and operator trial-and-error aortograms (83.7% vs. 52.3% vs. 42.1%, respectively, p=0.001). This finding was equally applicable in CoreValve THVs (p=0.036) and Edwards SAPIEN THVs (p=0.02).
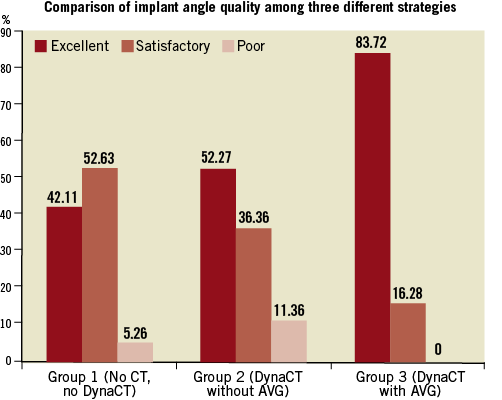
Figure 4. Comparison of implant angle accuracy across the three different strategies.
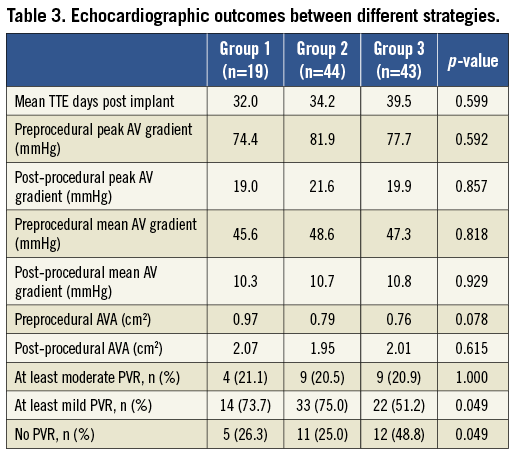
One hundred patients completed 30-day TTE follow-up. 19% of patients had at least moderate PVR on follow-up; 66% had at least mild PVR. Only 34% of patients had no PVR at 30 days. The AVG strategy was significantly more likely to lead to no PVR at all, compared to patients in Group 2 or Group 1 (48.8% vs. 25.0% vs. 26.3%, respectively, p=0.049) (Table 3). However, the three groups differed significantly in the THV type and operator experience. To mitigate the confounders, correlation was made between implant angle quality and PVR. There was a strong correlation between the implant angle quality and PVR (Table 4).
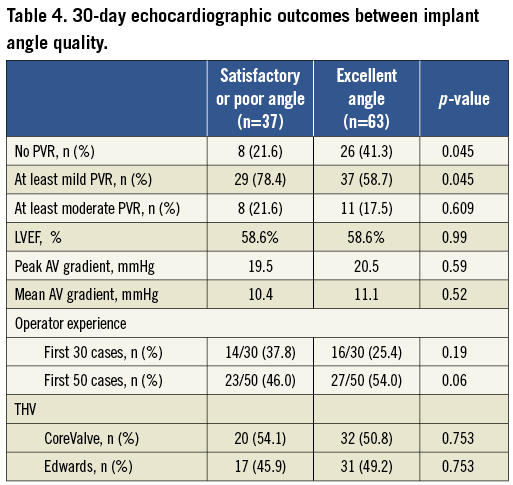
An excellent implant angle was significantly more likely to be associated with no PVR than satisfactory or poor implant angles (41.3% vs. 21.6%, respectively, p=0.045). There was no statistical difference between the excellent and non-excellent implant angle cohorts with regard to operator experience and THV type (Table 4). The proportion of excellent angles was numerically higher after the first 30 cases of TAVR than during the initial 30 cases but this did not reach statistical significance (47/70 vs. 16/30, p=0.19). There was also no statistical difference in the proportion of no PVR between the first 30 cases and the subsequent 70 cases (9/30 vs. 25/70, p=0.58).
Discussion
This study demonstrates that strategies to improve implant angle may be clinically relevant –a better implant angle may be an important factor in reducing PVR on follow-up. This study also demonstrates the enhanced accuracy of AVG in both CoreValve and Edwards SAPIEN THVs over stand-alone DynaCT.
This study confirmed the importance of rotational angiography in TAVRs. In Group 1, multiple aortograms were needed; despite this, the final implant angle chosen was still suboptimal. In most published series, different radiologists or cardiologists at various time points assessed MSCT data with different machines from DynaCT workstations. This study employed a novel method to minimise confounders –datasets from preprocedural MSCT and intraprocedural DynaCT on each patient were entered into the AVG workstation. Angles were then generated automatically for each modality from the AVG workstation, taking away any human interpretation. This theoretically negated some potential bias, e.g., inexperienced radiologists/cardiologists interpreting MSCT leading to inaccurate predictions. On the perpendicular line, there was a 7.38° (±16.83°) caudal difference between DynaCT and MSCT in the optimal coaxial angle. This difference was thus almost entirely attributable to patient positioning. In centres with no access to DynaCT, every effort should be made to develop patient positioning protocol to enhance the prediction accuracy of the MSCT. From our experience, a few degrees will not be discernible to the naked eye on aortography. The extent of off-axis degrees that would translate to clinical implications remains to be defined.
The AVG strategy was significantly more likely to provide an excellent implant angle compared with DynaCT or trial-and-error aortography. The incremental improvement in the accuracy of AVG over DynaCT was a surprising finding. Both operators had a >50-case experience and it was unlikely that inexperience in choosing an acceptable angle from DynaCT was a factor. Additionally, Group 2 patients underwent TAVRs at a time prior to the availability of AVG and every effort was made to select a perfect angle with the available technology. It may be concluded that AVG-generated angles significantly better operator-generated angles even in experienced hands. In centres with DynaCT, AVG should be used in conjunction with DynaCT.
With regard to safety, there was a trend towards higher radiation and contrast use despite fewer aortograms and shorter fluoroscopy time with the AVG strategy. Body mass index was not compared, and this may account for the higher radiation dose despite shorter radiation exposure. There were no catastrophic complications such as coronary ostia obstruction or embolised THV, complications directly attributable to incorrect positioning along the aorto-ventricular axis. The six patients in the entire cohort who required re-ballooning or snaring of the THV all had excellent or satisfactory implant angles, which would suggest other factors may be responsible.
The inclusion of CoreValve THVs in this study was important. Whilst some may dismiss that CoreValve THVs are repositionable, an optimal perpendicular angle is still mandatory for such positioning to be effective and accurate. Optimal positioning of CoreValve THV may be more important than Edwards SAPIEN THV owing to the different designs.
The main finding from this study is the impact of an excellent implant angle –excellent implant angles led to significantly less PVR than other implant angles, independent of operator experience and THV type. There is as yet no recognised measure of operator experience and its direct correlation with clinical outcomes, but this study had adopted recommendations from a recent consensus statement21 of using an institutional experience of more than 30 TAVRs as the cut-off. The influence of operator experience may have been underestimated in this small study and we do feel that more experienced operators are likely to have less PVR possibly with higher implant positions. Optimisation of implant angles would not have been the only factor in the reduction in PVR.
Given the adverse effects of PVR, its reduction may have significant implications. Whilst previous data only highlighted factors such as aortic valve sizing using 3-D imaging and leaflet calcification as factors impacting on PVR, this study suggests that optimising implant angles, thus ensuring a perpendicular aorto-ventricular axis, may also play a role. All these factors probably interact, but it remains unclear which of the three factors is the most important. We suspect THV sizing is probably more important than the aorto-ventricular placement. A perfectly sized valve will still lead to PVR if incorrectly positioned, just as a perfect AV-position could still be associated with severe PVR if the THV is undersized. Our study suggested this, as some excellent implant angles still led to 2/4 PVR. Perhaps analysis of a larger cohort will be necessary, or THV sizing may be a more important factor for a more severe degree of PVR.
There is now emerging data regarding the inadequacy of 2-D imaging in TAVR. MSCT is now arguably the gold standard for preprocedural assessment in TAVR, and 3-D echocardiography significantly improves understanding22. This study extends the idea of using 3-D imaging to the implant phase. In the coronary era of percutaneous interventions, 3-D fluoroscopy was not as important. However, for the naturally oval complex aortic valve structure with highly variable aortic root orientation, 2-D echocardiography and fluoroscopy are proving insufficient. Our study suggests 3-D guidance during THV implants may have important clinical implications. Given the impact of even mild PVR on mortality, larger studies may cement the importance of the optimal implant angle. No amount of THV manipulation will be accurate if the implant angle is flawed. In addition to future endeavour to optimise valve sizing, efforts to optimise fluoroscopic angle during implants may also be important.
Study limitations
This was a post hoc analysis in a prospective observational study with all the attendant weaknesses inherent in this study method. The three patient groups differed in the time of TAVR, and operator experience was a likely confounder in the PVR outcome. We attempted to minimise this by correlating implant angle with PVR. Nonetheless, operator experience very likely plays a role, although there is currently limited data in this area. Other factors such as THV undersizing or aortic leaflet calcification affect PVR and the study did not assess for these. All patients underwent the same THV sizing algorithm, and potential THV sizing errors may not account for differences within groups or implant angle quality. Assessment for differences in the degree of leaflet calcification between groups, echocardiographically or with MSCT, would have been useful. PVR was only reported qualitatively, in line with most current published literature. PVR assessment remains controversial as highlighted in the recent guidelines23 on TAVRs. The use of a continuous variable quantification, e.g., regurgitant volume, may augment the benefits of improved implant angles even more.
Conclusion
DynaCT with AVG provides a significantly more accurate prediction of the ideal perpendicular implant angle than DynaCT alone or MSCT. Optimisation of implant angle quality was associated with a reduction of PVR. In addition to improving aortic annulus sizing, future research should investigate strategies such as AVG to optimise implant angles. This may improve clinical outcomes.
Acknowledgements
We are grateful to Dr Jin Lin Fu, MBBS, PhD, for his statistical analysis and to our research staff and nurses at The Prince Charles Hospital.
Conflict of interest statement
D. Walters is a proctor for Edwards Lifesciences, consultant to Medtronic Inc. and Siemens, and principal investigator for SOURCE ANZ registry. The other authors have no conflict of interest to declare.
Figure 1. Left, stand-alone DynaCT generates a 3-D aortic reconstruction after removal of bone and soft tissues. Centre, gridlines are available to facilitate lining up the three cusps with manual rotation. Right, check aortogram of such a predicted angle.
Figure 2. AVG automatically generates an implant angle where all three cusps are lined up and equidistant to each other; the angle closest to straight AP would be selected by the algorithm. Coronary ostia are identified.
Figure 3. Examples of excellent, satisfactory and poor implant angles. Top panel, Edwards SAPIEN THV; bottom panel, CoreValve THV. Top left, excellent; top middle, satisfactory; top right, poor. Bottom left, excellent; bottom middle, satisfactory; bottom right, poor.
Moving image 1. DynaCT without AVG. The operator rotates the reconstructed 3-D images until all three cusps align to the operator’s satisfaction.
Moving image 2. DynaCT with AVG. Stylised representation of AVG analysis. AVG automatically generates the perfectly perpendicular angle (see red circle below aortic annulus which the AVG algorithm deems perpendicular to the aortic axis). Note the equal distance between the three cusps.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. DynaCT without AVG. The operator rotates the reconstructed 3-D images until all three cusps align to the operator's satisfaction.
Moving image 2. DynaCT with AVG. Stylised representation of AVG analysis. AVG automatically generates the perfectly perpendicular angle (see red circle below aortic annulus which the AVG algorithm deems perpendicular to the aortic axis). Note the equal distance between the three cusps.

