Introduction
With transcatheter aortic valve implantation (TAVI) rapidly spreading its indication to lower-risk and younger patients, reliable and reproducible guidance on how to orient the transcatheter heart valve (THV) to avoid coronary overlap and improve haemodynamic performance becomes an urgent need. It has recently been suggested that the orientation of the neo-commissures during the navigation of the delivery system could be correlated with their final position in the aortic annulus and, consequently, modified to improve final THV orientation1. However, no previous study has considered the effect of different aorta angulations and how to add this variable to a neo-commissure alignment model, and none have reported the feasibility of neo-commissure alignment with the Portico™ system (Abbott Structural Heart, Santa Clara, CA, USA). Therefore, this article aims to provide step-by-step recommendations on how to align the Portico THV, in a reproducible and standardised way, and to present the initial results obtained in patient-specific 3D printed aortic models.
Methods and results
In order to validate the proposed model, computed tomography scans from patients with distinct aorta angulations (standard, horizontal, and anteriorised) were used to print three different 3D models. The steps to develop the models are described in Figure 1. To evaluate neo-commissure alignment feasibility, the fully resheathable self-expanding Portico THV was used. This device has intra-annular leaflets, large stent cell geometry, and a low-profile delivery system, which provides remarkable flexibility and smooth tracking. It has three markers indicating the neo-commissures (Figure 1C), thus facilitating their identification and reorientation. To align the neo-commissures, we followed the steps described in Figure 2, Moving image 1, and Moving image 2. Neo-commissures were considered aligned if the degree of deviation, having the native commissures as a reference, was between 0° and 15º.
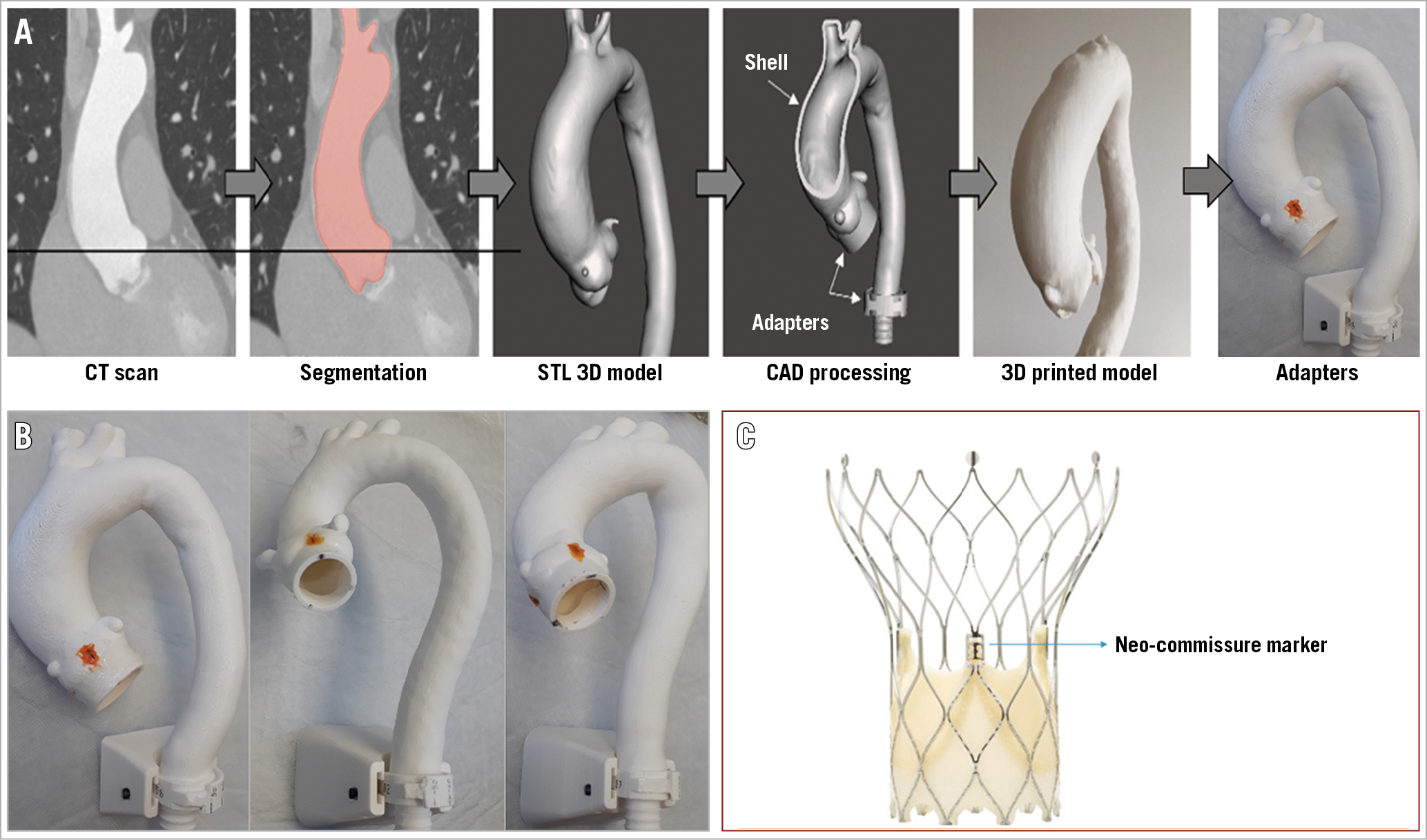
Figure 1. Steps to develop the different 3D models. A) Patient-specific CT scans were segmented using a free and open-source 3D Slicer software. The segmented aorta was exported as an STL 3D model and processed using the Meshmixer software (Autodesk, San Rafael, CA, USA). A shell was designed, representing the aortic wall. 3D models were printed on the Prusa I3 MK3 3D printer, with plastic filament polylactic acid material. Native commissures were marked using radiopaque markers and additional adapters were attached to the model. B) 3D models printed in different aorta angulations (horizontal, standard, anteriorised). C) Portico neo-commissure markers.
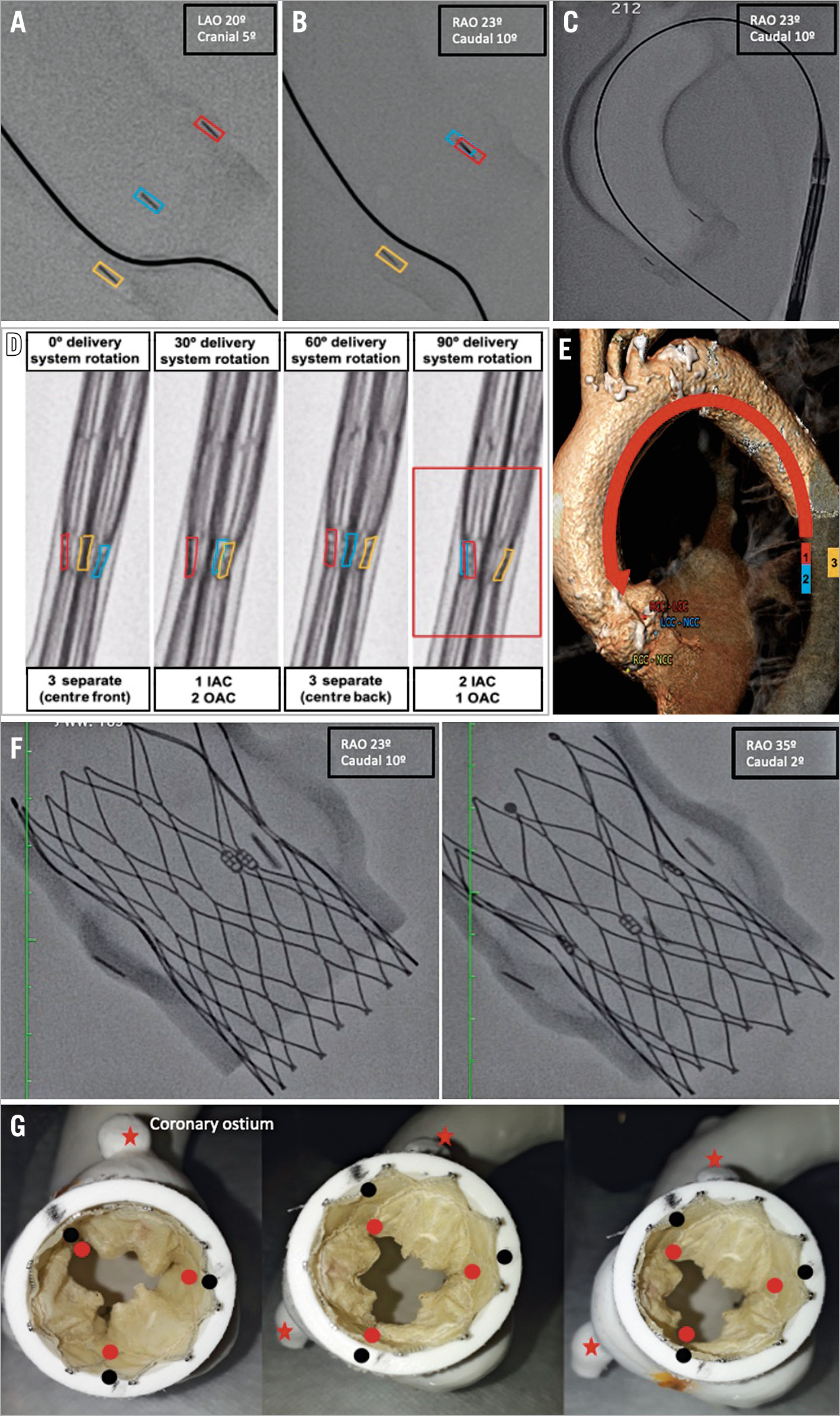
Figure 2. Neo-commissure alignment concept. Three native commissure markers were separately identified (A). A fluoroscopic projection depicting two native commissures in overlap in the inner aorta curve (IAC) and one isolated in the outer aorta curve (OAC) was set (B). Keeping this projection, the Portico delivery system was advanced to the descending thoracic aorta (C). The three neo-commissure markers were oriented similarly to the native ones (two in the IAC and one in the OAC) by rotating the delivery system clockwise until the desired position (red box) was achieved (D). This concept considers the premise that structures localised in the descending aorta’s medial aspect will be projected in the IAC once the delivery system is advanced (E). Standard fluoroscopic projection was resumed (avoiding parallax effect), the delivery system advanced (keeping the employed rotation), and the THV deployed as usual. At the end of the implant, the X-ray tube was rotated to check the final alignment in different projections (F). Final neo-commissure alignment evaluation in the benchtop model. Since the deviation between native commissures (black dots) and neo-commissures (red dots) was <15º, the implant was considered aligned (G).
To evaluate the proposed concept’s reproducibility and consistency, two different operators performed five deployments on each model, counting 15 deployments per operator, 30 deployments in total. Neo-commissure alignment was obtained in all tests, which equates to 100% success (Figure 2F, Figure 2G). The deviation between neo-commissures and native commissures and the degrees of rotation employed in the delivery system are shown in Supplementary Table 1. Overall, the delivery system was rotated clockwise between 70° and 120º, varying according to aorta angulation.
Discussion
With TAVI expanding rapidly to younger and lower-risk patients, concerns about future coronary re-access, THV durability, and redo TAVI feasibility have become particularly relevant. Among the factors related to THV durability and coronary re-access, THV orientation has received growing interest since it is a potentially modifiable factor. It has been demonstrated that an asymmetrical or non-aligned THV deployment may result in coronary obstruction, challenging coronary re-access, poor haemodynamic performance, intraluminal thrombosis, accelerated leaflet deterioration, and reduced prosthetic functional life2. Ochiai et al reported unfavourable coronary access in 25.8% and 34.8% (for the right and left coronary, respectively) of patients submitted to an Evolut™ R/PRO (Medtronic, Minneapolis, MN, USA) implant, and in 8.1% and 15.7% of those who received a SAPIEN 3 (Edwards Lifesciences, Irvine, CA, USA)3. Similarly, Tang et al1 retrospectively reported overlap with at least one coronary artery in 30% to 50% of patients. For the Evolut THV, the authors achieved significantly less overlap if the Evolut “hat” was located at an outer curve (OC) or centre front (CF) position in the descending aorta. Despite the relevance of these pioneer studies, the impact of different aorta angulation on final THV orientation was not considered. Even with the “hat” position modification proposed by Tang, overlap with one or both coronaries was still present in 20.2% of the cases. Furthermore, no study evaluating Portico neo-commissure alignment feasibility is available so far.
Taking into consideration these facts, the present article is innovative since:
1. We describe in a detailed way how to develop a patient-specific 3D printed aortic model, which provides a reliable TAVI simulated environment.
2. We provide step-by-step recommendations on how to reorient the Portico delivery system to reach final neo-commissure alignment, which proved to be consistent and reproducible, even in different aorta angulation models.
Using these models and the steps described above, we demonstrated an excellent correlation between the neo-commissure marker’s orientation in the descending aorta and their final position in the aortic annulus. Therefore, simple delivery system rotational manoeuvres, concurrently with native and neo-commissure overlap, led to a satisfactory final THV orientation. Using these same steps, with slight adaptations, we extrapolate that neo-commissure alignment could also be reached in real-life TAVI deployments (Supplementary Figure 1).
Limitations
The results reported here apply to experimental models and are limited by lack of clinical validation. We are currently performing a prospective clinical study to validate this concept in real-life TAVI procedures.
Conclusion
Neo-commissure alignment is a crucial issue given that misalignment has been associated with post-procedural challenging coronary re-access and faster THV deterioration. Experimental tests using 3D printed aortic models demonstrated the feasibility and effectiveness of Portico neo-commissure alignment. The proposed orientation steps were simple and straightforward, with success in all deployments performed.
|
Impact on daily practice With TAVI moving to lower-risk and younger populations, reliable and reproducible guidance on how to reach neo-commissure alignment becomes an urgent need. Patient-specific 3D-printed aorta models are useful for studying the Portico THV alignment technique. Since the realignment steps described here were successful in providing neo-commissure alignment, they could be translated, with slight adaptations, to a real-life scenario, contributing to improving THV orientation and facilitating coronary re-access. |
Conflict of interest statement
A.P. Tagliari reports a research grant from CAPES-Brasil (Finance Code 001). M. Miura reports being a consultant for Japan Lifeline. E. Ferrari reports being a consultant for Edwards and receiving research support/grants from Edwards, Medtronic and Somahlution. F. Maisano reports research support/grants from Abbott, Medtronic, Edwards, Biotronik, Boston, NVT and Terumo, and consulting fees/honoraria from Abbott, Medtronic, Edwards, SwissVortex, Perifect, Xeltis, Transseptal Solutions, Cardiovalve and Magenta, royalty income/IP rights from Edwards, and being a shareholder in CardioGard, Magenta, SwissVortex, Transseptal Solutions, 4Tech, and Perifect. M. Taramasso reports being a consultant for Abbott, Boston and 4Tech, and receiving consulting fees from Edwards, CoreMedic, SwissVortex and Mitraltech. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Native commissure and neo-commissure alignment steps.
Moving image 2. The neo-commissure alignment concept step by step.

