Abstract
Aims: We evaluated a novel motion-compensating 3D reconstruction technique applied to rotational angiography (R-angio) which produces MSCT-like images for evaluation of implanted TAVI prostheses without requiring rapid pacing.
Methods and results: Fifty-one consecutive patients were retrospectively identified who were evaluated with rotational angiography (R-angio) using the Siemens Artis zee angiographic C-arm system after TAVI with a Medtronic CoreValve prosthesis. A novel 3D image reconstruction technique was applied which corrects for cardiac motion. CoreValve frame geometry was evaluated according to the same protocol for MSCT and R-angio at the level of: 1) the inflow, 2) the nadirs, 3) central coaptation, and 4) the commissures. The native aortic annulus dimensions were measured at the nadirs of the three leaflets. Sizing ratio, prosthesis expansion and frame ellipticity were assessed. Good quality 3D reconstructions were obtained in 43 patients (84%) and failure was predictable prior to reconstruction in six of the other seven patients (superposition of radiographically dense object n=4, obesity n=2). Prosthesis inflow ellipticity and expansion were correlated with implantation depth (respectively r=-0.46, p<0.01, and r=0.61, p<0.001). Aortic regurgitation grade ≥2 was associated with greater prosthesis ellipticity at the level of central coaptation (median [25th-75th percentile]: 1.15 [1.10-1.20] vs. 1.08 [1.06-1.12], p=0.009). The inter-observer, inter-modality (MSCT, R-angio) variability in measurement at the level of coaptation for minimum diameter, maximum diameter and area were all low (respectively, mean ±SD:1.2% ±1.2; 1.7% ±1.8 and 2.0% ±1.3).
Conclusions: R-angio with motion-compensated reconstruction offers new possibilities for evaluation of the post-implantation geometry of percutaneous structural heart prostheses and the potential clinical effects.
Introduction
Transcatheter aortic valve implantation (TAVI) improves symptoms and prognosis in patients with aortic stenosis and at high surgical risk1. In keeping with the evolution of a new technique there is a tendency to perform TAVI in younger patients who have less comorbidity2. One prerequisite for such a step is the long-term durability and integrity of TAVI prostheses.
Distortion of nominal prosthesis geometry may contribute to acute dysfunction and reduce durability. Dense calcification may cause inadequate frame expansion, malapposition or, in extreme cases, eccentric pinching of the frame resulting in aortic regurgitation (AR)3. If it is detected, an attempt may be made to correct the asymmetry/malapposition with judicious post-dilatation of the frame4. However, due to the mass of overlapping frame struts and the limitations of 2D cine-fluoroscopy it may be difficult to detect frame asymmetry and underexpansion. MSCT allows 3D evaluation of frame geometry, but it is not available in the catheter laboratory. Rotational angiography (R-angio) may provide multiple angiographic views, but motion artefacts resulting from the poor temporal resolution severely degrade 3D images.
Acquiring R-angio during rapid ventricular pacing reduces motion artefacts by effectively causing ventricular standstill. Novel approaches may correct for frame motion during the image reconstruction phase without requiring rapid pacing5.
We investigated the image quality obtained with R-angio motion-compensated reconstruction of the implanted Medtronic CoreValve (Medtronic, Minneapolis, MN, USA) frame and the clinical and anatomical correlates of 3D frame geometry.
Methods
PHANTOMS
In order to compare the nominal dimensions obtained from MSCT and R-angio, a CoreValve frame of each size (inflow diameter 26 mm, 29 mm and 31 mm) was imaged ex vivo, motionless and uncrimped in a water bath at 38 degrees Celsius, by both R-angio and MSCT followed by 3D reconstruction and measurements as described below.
PATIENTS
The study was approved by the institutional review board. Written informed consent was obtained from each patient. Fifty-one consecutive patients were retrospectively identified who were evaluated with R-angio after TAVI with a CoreValve. ECG-triggered MSCT performed after TAVI was available in 11 of these patients.
R-ANGIO ACQUISITION AND 3D RECONSTRUCTION
R-angio was performed after TAVI using the Artis zee angiographic C-arm system (Siemens AG, Forchheim, Germany), which has a 20×20 cm detector size and isotropic pixel length of 180 µm. One hundred and thirty-three projection images were acquired in 5 s along a 198° arc (99° right anterior oblique to 99° left anterior oblique view), during a breath hold, at a detector entrance dose of 0.36 μGy per frame. The ECG signal was recorded. From the images a motion-compensated 3D R-angio data set was reconstructed (reconstruction prototype; Siemens AG, Forchheim, Germany), with a matrix of 256×256×256 and 0.5 mm3 voxel size.
A novel 3D image reconstruction software that is available only as a prototype was applied5,6 (Figure 1), which estimates cardiac motion from the acquired images and compensates for it in the reconstruction step. Firstly, a 3D reference image is generated by ECG gating at a pre-selected heart phase. The 3D image is optimised by iterative motion estimation and compensation of an unknown 4D deformable motion vector field such that the intermediate images register well to the initial reference image. The 4D equation does not assume periodicity in the motion and all projection images are used. For computation of the initial reference image, ECG gating selects projection images at 40% of the RR-interval weighted by a cos4 function. The 3D reference image is sensitive to non-periodic motion caused by, for example, arrhythmia and residual breathing motion. An optional motion pre-correction resolves this problem6. An iterative optimisation procedure searches for affine motion parameters for each ECG-gated projection image such that the forward projection of the motion pre-corrected 3D reference image coincides with the acquired 2D projection views. Noisy images obtained from obese patients or overlaying structures, e.g., the X-ray shadow of pacemaker electrodes, may cause false registration of the intermediate 3D image to the initial 3D reference image. In most cases, this may be easily corrected by manual volume cropping and grey-scaling of the reference image.
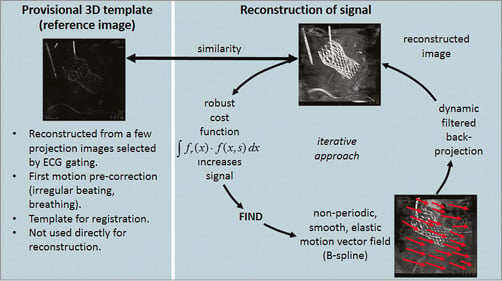
Figure 1. Overview of the methods used for the 3D reconstruction of objects that are in motion. The first part (left) generates the structure of the object which is then used to verify the signal accumulated by the second part (right).
MSCT ACQUISITION AND IMAGE PROCESSING
The MSCT acquisition method has been described before4. In brief, the acquisition was performed using a 128-slice dual-source CT (SOMATOM Definition Flash; Siemens AG, Forchheim, Germany) in spiral scan mode with a variable table speed adjusted to the heart rate and detector collimation 2×64×0.6 mm and a z-flying focal spot (Z-sharp®; Siemens AG). The scan range was set from the top of the aortic arch to the diaphragm. 80-100 ml of iodinated contrast was used. Single-segmental reconstructions with slice thickness 1.5 mm were made in end systole. The radiation doses ranged from 8 to 20 mSv.
EVALUATION OF THE AORTIC ANNULUS PRE-TAVI
Calcification of the aortic leaflets was measured as Agatston score on non-contrast MSCT4. On contrast MSCT a short-axis view of the aortic annulus was defined in the LVOT where the most caudal attachments of the three aortic leaflets were simultaneously and proportionately in view7. At the level of the aortic annulus the following measurements were obtained: maximum diameter (Dmax), minimum diameter (Dmin), perimeter, area.
INDEPENDENT AND BLINDED EVALUATION OF THE COREVALVE FRAME
The measurement of the frame geometry by MSCT and R-angio was performed by two different observers blinded to each other’s analysis and on separate workstations, but according to the same protocol8. Standard MSCT workstations (MMWP; Siemens AG) were used to obtain short-axis images of the CoreValve frame at four levels: 1) the inflow, 2) the nadirs of the new leaflets, 3) central coaptation of the leaflets, and 4) the commissures. At each level, orthogonal smallest (Dmin) and largest (Dmax) diameters, area and perimeter were measured on R-angio and also on MSCT when available (Figure 2). Sizing ratio was calculated as nominal prosthesis inflow perimeter/native annulus perimeter. Prosthesis expansion was calculated as measured/nominal frame perimeter at the inflow, and frame ellipticity was calculated as Dmax/Dmin at the various levels.
` 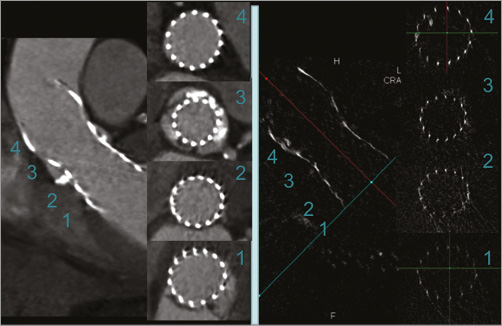
Figure 2. Evaluation of the implanted Medtronic CoreValve frame with multislice computed tomography (left panel) and rotational angiography (R-angio) with motion-compensated 3D reconstruction (right panel). The R-angio was acquired at the end of the TAVI procedure and the MSCT one week later in the same patient. Short-axis views are shown at the level of the inflow (1), the nadirs (2), central coaptation (3), and the commissures (4). At each level, the orthogonal smallest (Dmin) and largest (Dmax) diameters, area and perimeter were measured.
GRADING OF IMAGE QUALITY
The image quality of the 3D reconstruction from R-angio was graded as acceptable if the 3D geometry of the CoreValve frame could be evaluated or poor if not.
EVALUATION OF AORTOGRAMS
The aortogram prior to catheter removal after TAVI was used to grade AR according to the method of Sellers9. The depth of frame implantation was measured from the frame inflow to the floor of the non-coronary sinus.
Statistical methods
Data are presented as a median (25th to 75th quartile) or mean±standard deviation (SD) as appropriate. Pearson’s or Spearman’s correlation coefficients were determined. Comparisons between patients with and without AR ≥2 were performed using the Student’s t-test or the Mann-Whitney U test. Paired comparisons between MSCT and R-angio were performed using the Student’s t-test or Wilcoxon’s signed-rank test. Difference plots were constructed according to the Bland-Altman method. Measurement variability between MSCT and R-angio was calculated at the level of leaflet coaptation as the absolute difference divided by the mean multiplied by 100. SPSS 17.0 (SPSS Inc., Chicago, IL, USA) was used. Statistical significance was defined as a two-tailed p<0.05.
Results
PHANTOM
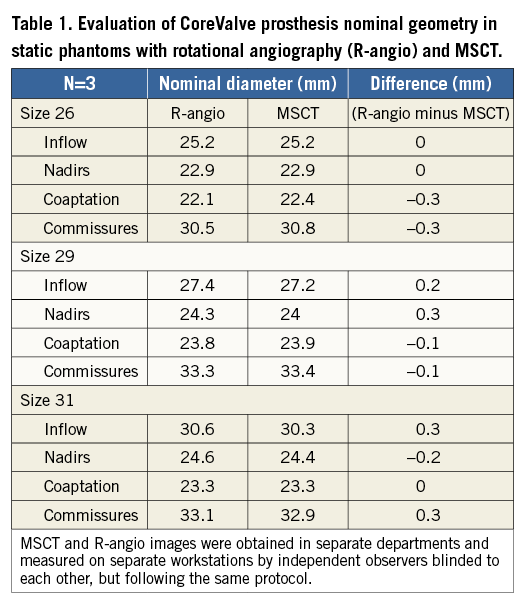
The measurements of nominal frame dimensions obtained by both MSCT and R-angio for each size CoreValve at the four levels of interest are shown in Table 1. Differences between the two imaging modalities were small (maximum difference=0.3 mm) and lower than the spatial resolution of either technique (0.5 mm).
CLINICAL CHARACTERISTICS AND IMAGE QUALITY ON R-ANGIO
Rotational angiography was performed post-TAVI in 51 patients, but one patient with a valve-in-valve was excluded. Clinical characteristics are shown in Table 2 and availability of data in Figure 3.
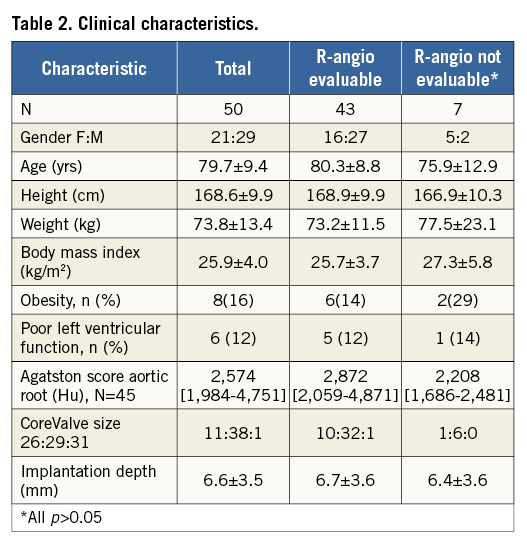
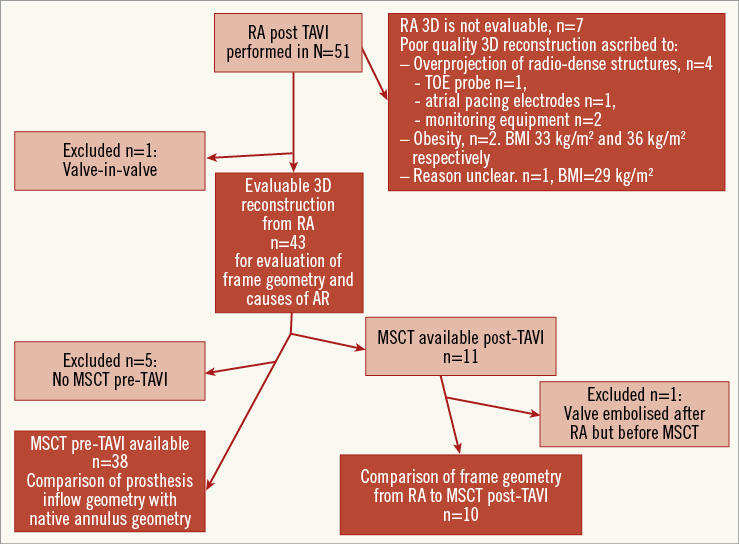
Figure 3. Data availability for the various steps (dark red boxes) in the analysis. AR: aortic regurgitation; BMI: body mass index; MSCT: multislice computed tomography; R-angio: rotational angiography; TAVI: transcatheter aortic valve implantation; TOE: transoesophageal echocardiography
Motion-compensated 3D image reconstruction clearly improved image quality overall (Figure 4), and was good quality in 43 cases (84%) and poor in seven (Figure 3). Steps in the reconstruction process which contributed to good image quality were: the initial reference image in the end-diastolic phase (in 84% of cases), motion pre-correction for the ECG-gated reference image (in 40%), manual selection of heart phase and volume cropping (in 14%).
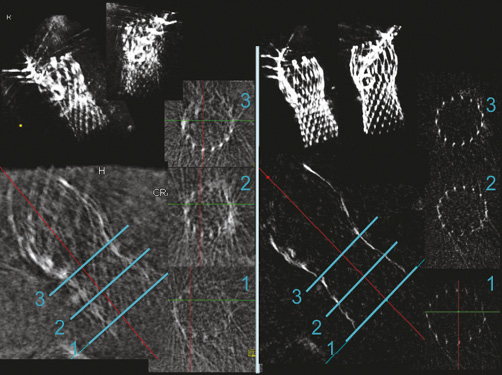
Figure 4. Example of rotational angiography with standard (left panel) and motion-compensated 3D reconstruction (right panel) of the implanted Medtronic CoreValve frame. 3D volume images are shown at the top. On the long-axis view, the blue lines through the frame indicate the levels of the corresponding short-axis view planes.
Poor image quality was ascribed to superposition of radiographically dense structures (n=4) or obesity (BMI >30 kg/m2, n=2) in six of seven cases (Figure 3). There were no significant differences in clinical characteristics between the patients with good and poor image quality on R-angio (Table 2).
COMPARISON OF FRAME GEOMETRY OBTAINED FROM R-ANGIO WITH MSCT
MSCT post-TAVI was available in 11 patients after a median of seven days (five to eight), but one patient was excluded because the prosthesis had embolised in the days between performing R-angio at the time of TAVI and MSCT (Figure 3). At the levels where the frame may be constrained by native tissue (inflow, nadirs) the frame Dmin and area were significantly smaller on R-angio than on MSCT, whereas there was no difference in Dmax (Table 3). At the levels where the frame was unlikely to be constrained by native tissue (coaptation, commissures), there was no difference in any of the frame dimensions between MSCT and R-angio (Table 3). The difference in Dmin between R-angio and MSCT at the inflow level was not correlated with implantation depth (r=0, p>0.05). The scatter and difference plots with limits of agreement of the MSCT and R-angio measurements are shown for inflow Dmin (Figure 5), and coaptation area (Figure 6).
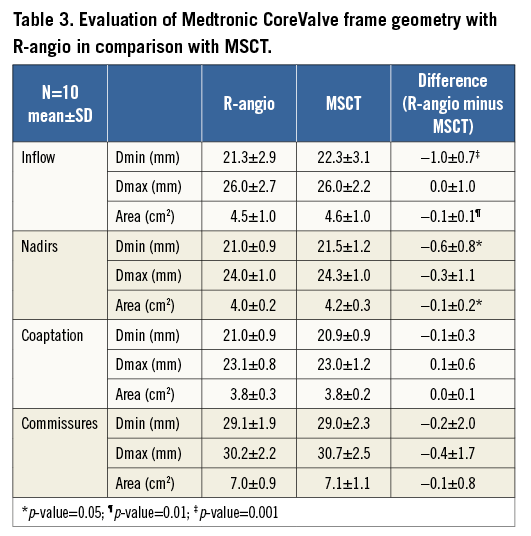
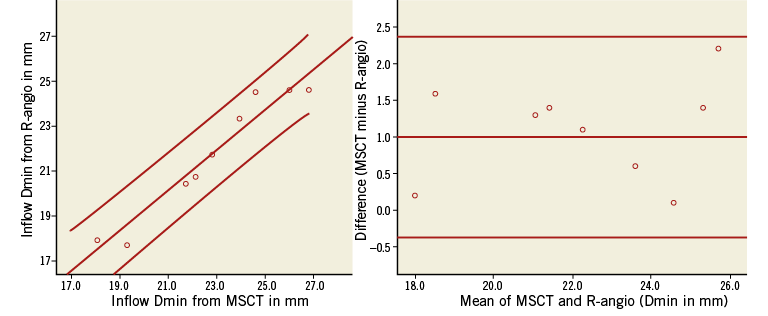
Figure 5. Scatter and difference plots of the minimum diameter at the inflow of the CoreValve as measured on MSCT and R-angio. The scatter plot (left) shows the linear regression line with 95% confidence interval. The difference plot (right) shows the mean difference and limits of agreement.
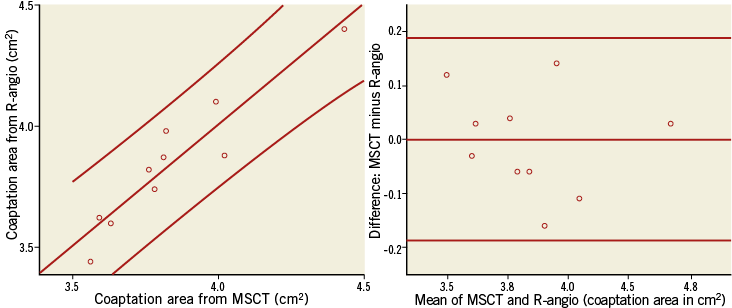
Figure 6. Scatter and difference plots of the cross-sectional area of the CoreValve at the level of coaptation as measured on MSCT and R-angio. The scatter plot (left) shows the linear regression line with 95% confidence interval. The difference plot (right) shows the mean difference and limits of agreement.
The inter-observer, inter-modality (MSCT, R-angio) variability in measurement at the level of coaptation for Dmin, Dmax and area were all low (respectively, mean ±SD: 1.2%±1.2; 1.7%±1.8 and 2.0%±1.3).
EVALUATION OF IMPLANTED FRAME GEOMETRY AND AORTIC REGURGITATION
AR ≥2 was seen in nine of 43 patients (21%) and was associated with greater prosthesis ellipticity at the level of central coaptation (median [25th to 75th percentile]: 1.15 [1.10-1.20] vs. 1.08 [1.06-1.12], p=0.009), whereas the difference was marginal at the nadirs (1.25 [1.15-1.35] vs. 1.17 [1.08-1.26], p=0.07) and not significant at the level of the inflow or the commissures (respectively, 1.18 [1.14-1.32] vs. 1.24 [1.15-1.31], p=0.2, and 1.05 [1.04-1.07] vs. 1.03 [1.02-1.05], p=0.1). Patients with AR ≥2 showed no difference in frame expansion (0.85 [0.79-0.91] vs. 0.83 [0.77-0.91], p=0.5) or with implantation depth (mean±SD: 6.6±3.9 mm vs. 6.9±1.7 mm, p=0.8).
In patients where a MSCT pre-TAVI was available (n=38 of 43), AR ≥2 was also associated with a lower sizing ratio (median [25th to 75th percentile]: 1.10 [1.0-1.12], n=7 vs. 1.14 [1.10-1.18], n=31, p=0.04).
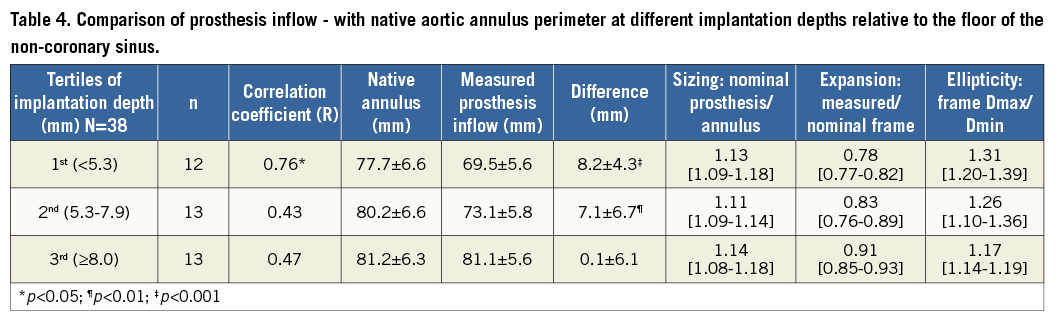
EFFECT OF IMPLANTATION DEPTH ON FRAME DIMENSIONS
Prosthesis ellipticity was correlated with implantation depth at the level of the inflow (r=–0.46, p<0.01) and the nadirs (r=–0.32, p<0.05) but not at coaptation or the commissures. Prosthesis expansion was also correlated with implantation depth (r=0.61, p<0.001).
For better evaluation of the effect of implantation depth on prosthesis inflow underexpansion and ellipticity, the patients were divided into three groups according to tertiles of implantation depth (Table 4). The sizing ratio was comparable in the three groups (median 1.13, 1.11 and 1.14, respectively, in the 1st, 2nd and 3rd tertiles of implantation depth) (Table 4). However, across increasing tertiles of implantation depth both underexpansion (median 0.78, 0.83 and 0.91) and ellipticity (median 1.31, 1.26 and 1.17) of the frame inflow were progressively less pronounced (Table 4).
Discussion
This study demonstrates the feasibility of using R-angio with motion compensation in the image reconstruction phase to obtain 3D images of the CoreValve frame. Good image quality was seen in 84% of patients without the use of rapid pacing. Poor image quality could have been prevented (three of seven patients) or predicted (another three of seven). Another study evaluating the role of R-angio with and without rapid pacing to assess the LVOT before TAVI in 56 patients obtained diagnostic image quality in non-obese patients without rapid pacing, whereas rapid pacing was required to ensure good image quality in obese patients10. A major difference with that study10, which made use of standard reconstruction, is that the diameter of the LVOT is on average 57 times larger than the struts of a CoreValve. That would explain why standard reconstruction resulted in good image quality of the LVOT10, whereas the novel motion compensation correction was required to obtain good images of the CoreValve in the present study.
Hardly any differences were seen between the measurements obtained from R-angio and those from MSCT of the nominal dimensions of the CoreValve frame in static phantoms. The nominal dimensions of the CoreValve frame in the present study were smaller at the inflow level than the measurements provided by the manufacturer11. Our protocol required measurement from the middle of a strut through the centre and to the opposite side on an axial image. The frame strut thickness is approximately 0.4 mm, although this is variable at different levels. It is possible that this measurement method would underestimate the external dimension by a total of 2×0.2 mm=0.4 mm. However, this would not be sufficient to explain the observed differences. Furthermore, the nominal dimensions at the level of coaptation and elsewhere were as expected, indicating that the measurements obtained at all levels are probably correct.
At the level of coaptation of the CoreValve leaflets or the commissures, which are not apposed to native tissue, there was no difference in the geometry as determined by R-angio and MSCT, and variation in measurement between the two modalities was low (≤2%), indicating a high degree of accuracy of the R-angio reconstruction. However, the dimensions of the frame were significantly smaller when assessed by R-angio when compared to MSCT at the inflow level and the nadirs, but not at central coaptation. Interestingly, this was due to a difference in Dmin but not in Dmax. It is possible that this difference may be explained by the cardiac cycle given that the MSCT reconstructions were systolic phase whereas the R-angio reconstructions were predominantly based on a diastolic phase reference image. It is recognised that the native aortic annulus Dmin may decrease in diastole due to opening of the anterior mitral leaflet11-13. Yet, in the present study the difference in MSCT and R-angio in Dmin at inflow level was not correlated with implantation depth, suggesting the need for an alternative explanation. The CoreValve frame consists of thermosensitive nitinol that is crimped in ice water and acquires nominal dimensions at normal body temperature. Conceivably, expansion may be delayed (anecdotally for 48 hours) in the areas where expansion is resisted by calcified tissues, i.e., at the level of the native annulus and leaflets. R-angio was acquired shortly after release of the CoreValve during TAVI whereas the MSCT was acquired one week later.
Both frame expansion and ellipticity varied according to depth of implantation. The inflow underexpansion and ellipticity were highest in patients with the highest implantations (<5.3 mm) and decreased progressively with increasing depth of implantation. A study which evaluated CoreValve frame expansion angiographically in 50 patients also reported that a higher level of implantation was associated with a greater degree of underexpansion11, but this question was not studied in another study evaluating the Edwards SAPIEN device14. These data indicate that it is the calcified native aortic leaflets that most constrain the CoreValve prosthesis rather than the native annulus. By implication, although the inflow was least constrained in patients with the deepest implantations, the level of the nadirs would be relatively more constrained and elliptical than in patients with higher implants. Simulation models based on finite element modelling suggest that, if severe, this may contribute to malcoaptation of the prosthesis leaflets15. In observational studies, increasing depth of implantation of the CoreValve was associated with higher grades of AR16,17. These data may indicate one potential mechanism by which deep implantations may contribute to AR post-TAVI.
In the present study the frame geometry at the inflow had no effect on AR grade. In contrast, ellipticity at the level of central coaptation was strongly associated with AR grade ≥2. Other studies discussed in the preceding paragraphs investigating the geometry of the CoreValve and causes of AR did not report this association and may not have done this comparison because the frame achieves, on average, dimensions close to the nominal at this level. However, frame ellipticity at this level may perturb leaflet coaptation and is a mechanistically plausible cause of AR. The observation is intriguing but requires verification and elucidation in larger studies.
ADVANTAGES AND LIMITATIONS
R-angio is available directly in the catheter laboratory and potentially allows the implanted frame to be studied in all TAVI patients. This is an advantage over MSCT where patients may develop complications post TAVI before MSCT may be performed, which may lead to bias in studies investigating the effects of frame expansion and ellipticity on outcome. Furthermore, frame geometry may also be studied during follow-up by bringing patients back to the catheter laboratory but without requiring vascular access or rapid pacing. On the other hand, MSCT has the advantage, with the addition of contrast, of allowing evaluation of frame apposition to the surrounding tissues, whereas this is currently not possible with R-angio. Accurate 4D reconstruction is not yet possible with R-angio. Although we studied only the CoreValve, the principles of reconstruction should apply to all large metal stents. The findings in this proof of concept study require verification in larger studies.
Conclusions
Evaluation of the 3D geometry of dense metal frames by R-angio with motion compensation during the reconstruction phase is feasible and accurate. A greater degree of underexpansion and a more elliptical shape of the CoreValve frame were inversely associated with implantation depth. AR ≥2 was associated with greater prosthesis ellipticity at the level of central coaptation. R-angio with motion-compensated reconstruction offers new possibilities for evaluation of the post-implantation geometry of percutaneous structural heart prostheses and potential clinical effects.
| Impact on daily practice 3D imaging with MSCT is now considered standard practice for planning transcatheter valve procedures and is increasingly used for evaluation of prosthesis geometry post implantation. The new 3D method of R-angio with motion-compensated reconstruction without rapid pacing has the advantage that it can be performed in all patients prior to leaving the catheter laboratory. It also introduces new possibilities for frame evaluation post implantation and at follow-up. Interesting associations seen between distortion of frame geometry at the level of leaflet coaptation and significant aortic regurgitation require verification in larger studies. |
Guest Editor
This paper was guest edited by Gerald Maurer, MD; Division of Cardiology, Department of Medicine II, Medical University of Vienna, Vienna, Austria.
Acknowledgements
We gratefully acknowledge the contribution of Patrick Geeve to this work.
Conflict of interest statement
P. de Jaegere is a proctor for Medtronic. G. Lauritsch and C. Rohkohl work for Siemens AG, Healthcare Sector, Forchheim, Germany. The other authors have no conflicts of interest to declare. The Guest Editor has no conflicts of interest to declare.

