Abstract
Aims: High quality three-dimensional imaging is one of the cornerstones in structural heart disease interventions. Current mainstream technology to acquire three-dimensional imaging utilises computed tomography or magnetic resonance imaging. Incorporation of these data with conventional angiographic images may not be sufficient. We describe a new imaging technique consisting of rotational angiography combined with rapid pacing to obtain real-time, high-quality, three-dimensional images in the catheterisation laboratory.
Methods and results: Rotational angiography is performed with breath holding and rapid pacing on a large format digital flat-panel angiographic system. During a 200 degrees rotation, 150 angiographic images are acquired in five seconds and automatically reconstructed in less than 30 seconds. This imaging technique was used in six patients (mean age 32±10 years) to guide structural heart disease interventions. No complications were associated with rapid pacing. This imaging technique allowed acquisition of high-quality, three-dimensional images with a low volume of contrast media. Volume renderings helped appreciation of the lesions and optimisation of the working views. Multiplanar visualisation allowed true orthogonal measurements of vascular diameter during the procedures.
Conclusions: The advantages of this imaging technique include rapid image acquisition and precise imaging of complex structures using low volume of contrast media.
Introduction
Structural heart disease intervention is a growing field of invasive cardiology and good quality imaging is one of the cornerstones for planning and performing these procedures. Single-plane angiography suffers from the intrinsic limitations of projective two-dimensional imaging, and does not offer three-dimensional volumetric appreciation needed to image complex and abnormal anatomies. Three-dimensional reconstruction images can be generated using computed tomography or magnetic resonance (MR) imaging. However, these imaging modalities are often associated with high radiation and contrast use or poor availability. Furthermore, incorporation of these data with conventional angiographic images may not be optimal, as the corresponding exams are usually performed prior to the intervention. Having access to real-time, three-dimensional imaging in the catheterisation laboratory is desired.
Rotational angiography with three-dimensional reconstructions can be performed using standard cine-fluoroscopy equipment and additional software. To obtain high quality images, vascular structure opacification need to be adequate during the whole rotation. Various methods have been described to transiently decrease cardiac output during interventional procedures such as aortic valvuloplasty, transcatheter aortic valves replacement (TAVR) or placement of stents1-3. Rapid ventricular pacing is the most commonly used technique and it is usually well tolerated.1 Therefore, we have developed a new imaging protocol combining rotational angiography with rapid ventricular pacing to perform real-time, three-dimensional imaging in the catheterisation laboratory.
Methods
Rapid pacing, rotational angiography with real-time, three-dimensional reconstruction performed in the catheterisation laboratory was used in six adult patients referred to our institution for structural heart disease interventions including pulmonary artery stenting (n=3), coarctation stenting (n=2) and percutaneous pulmonary valve replacement (n=1). No patient suffered from decreased renal function or iodine allergy. Written informed consent was obtained from all patients before cardiac catheterisation.
Imaging acquisition technique
Rotational angiographies over 200 degrees from lateral to lateral (right anterior oblique 100° to left anterior oblique 100°) were performed in all six patients with breath holding and rapid pacing, on a large-format, digital, flat-panel angiographic system (GE-Healthcare Innova™ 4100IQ, Waukesha, WI, USA). The images were acquired in a 40 cm field of view, which deliver 84.1 µGy/frame (manufacturer’s bench test).
Rapid ventricular pacing
Before the image acquisition, a transvenous pacing catheter was placed in the right ventricular apex under fluoroscopic guidance. Satisfactory lead position was confirmed by testing the minimal pacing threshold. The output was set to a level that was at least triple this threshold. The blood pressure was invasively monitored using an arterial line. Pacing was performed at a rate of 180 beats per minute (bpm) with electrocardiographic and hemodynamic monitoring. The objective was to reduce the cardiac output in order to decrease the vessel motion, slow the contrast wash-out and thus enhance the imaging quality. There was no pre-specified target for the magnitude of drop in blood pressure.
Contrast injection and images acquisition
As soon as the blood pressure started to drop, diluted contrast media (105 cc) was injected through a power injector in a bolus consisting of 60% (63 cc) Iodixanol-320 contrast agent (Visipaque™, GE Healthcare Canada Inc, Mississauga, Ontario, Canada) and 40% (42 cc) saline. The contrast was injected over six seconds with a rate of 17 cc per second. A total of 150 angiographic images (30 frames/sec) were acquired during five seconds, starting one second after injection to allow the contrast media to fill the target vessel. Breath holding was used in all cases. The catheter used for contrast media injection was mainly a 6 Fr or 7 Fr pigtail catheter (Cordis, Johnson and Johnson, Miami Lakes, FL, USA) positioned at the area of interest.
Three-dimensional image reconstruction
Images acquired with rotational single plane angiography were automatically forwarded to a dedicated workstation for three-dimensional reconstruction and analysis (Innova3D software and Advantage Workstation, GE Healthcare, Chalfont St Giles, United Kingdom). Various renderings of the three-dimensional images such as volume rendering, maximum intensity projection and multiplanar reformations were performed in less than 30 seconds of processing time and presented during the procedure. Using the computer workstation tools, the reconstruction parameters were adjusted for different tissue densities. Manual elimination of unwanted tissues (bones, liver, diaphragm, and other vessel than the target) could be performed if deemed necessary to better visualise the structure of interest. Multiplanar visualisation allowed measurement of true orthogonal diameters of vascular structures with no need for a calibration catheter.
Results
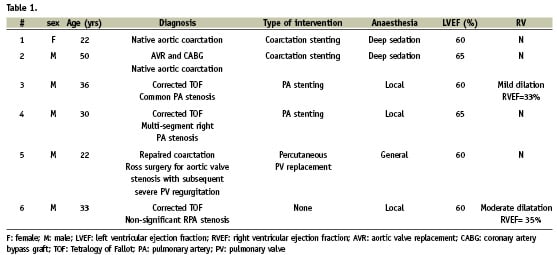
Table 1 summarises the baseline characteristics and interventions of the six adult patients (mean age 32±10, range 22-50 years). All patients had hemodynamic assessment with transvalvular or trans-stenotic gradient measurement. Five of the six patients proceeded to have an interventional procedure. The remaining patient was referred for stenting of a left pulmonary artery stenosis in a background of corrected Tetralogy of Fallot. However after hemodynamic assessment, which demonstrated a maximal gradient of only 15 mmHg, and adequate imaging of the area of interest showing an artery diameter measurement of 17x19 mm, we decided not to proceed with pulmonary artery stenting.
Rotational angiography was performed with rapid pacing at baseline with acquisition of high-quality, three-dimensional images in all patients. A low volume of contrast media was used (63 cc per acquisition). Post-interventional imaging was also performed using the same protocol of image acquisition and reconstruction. No complications were associated with rapid pacing including no ventricular arrhythmia or hemodynamic compromise. Blood pressure recovered rapidly to the baseline value after pacing interruption.
Representative case nr.1: extreme aortic coarctation stenting
A 22 year old woman with native aortic coarctation diagnosed since age three was referred for percutaneous stenting. She had refractory hypertension, despite three different anti-hypertensive medications. Coarctation was extremely severe at magnetic resonance imaging and was localised at 19 mm below the origin of the left subclavian artery. Collateral circulations were well developed around the obstruction.
By invasive assessment, maximum systolic gradient across the coarctation was measured at 30 mmHg. A rotational aortogram was performed (Figure 1 and Video 1) with breath holding and rapid pacing. Three-dimensional multi-planar visualisation (Figure 2a and Video 2a) allowed true orthogonal measurements of vascular diameter during the procedure: 27.4 mm (28 mm at MR angiography assessment) at the largest part of the descending aorta; 11.6 mm (11.4 mm at MR angiography assessment) at 14 mm from the subclavian ostium. Volume renderings (Figure 2b and Video 2b) helped appreciation of the lesions and optimisation of the working views.
Under deep sedation and guidance of these images, a covered NuMED CP stent 8 ZIG 39 mm long (NuMED Inc., Hopkinton, NY, USA) mounted on a BIB balloon 18 mm x 4 cm (NuMED) was implanted at 8 atmospheres without complications. The majority of the collaterals disappeared at the conclusion of the procedure with no residual gradient in the aorta. (Figure 3, 4a-4b, Video 3). At one year, the blood pressure was within normal limits, with only two of the three previous antihypertensive medications at half dose.
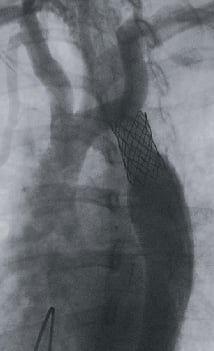
Figure 3. Still frame from the rapid pacing rotational angiography post-stent implantation. Important reduction of the collateral circulation can be appreciated.
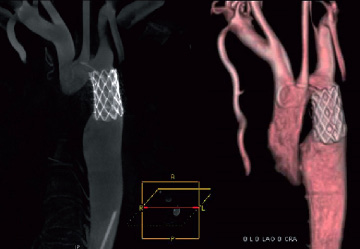
Figure 4. A: Maximum Intensity Projection (MIP) post-stent implantation (sagittal view). B: Volume rendering (antero-posterior view) post-stent implantation. These two images from three-dimensional reconstructions show the covered stent well expanded without residual waist.
Representative case nr.4: multi-segment pulmonary artery stenosis stenting
A 30 year old man was admitted for percutaneous treatment of multi-segment right pulmonary artery stenosis, visualised on magnetic resonance imaging, with right lung hypoperfusion (39% vs. 61% for the left lung at the nuclear medicine test). He was born with an extreme Tetralogy of Fallot, consisting of a large ventricular septal defect, extreme aortic dextroposition and pulmonary atresia. At five years of age, his congenital heart disease was corrected with closure of the ventricular septal defect and insertion of a Hancock bio-prosthetic valved conduit (Medtronic, Minneapolis, MN, USA) between the right ventricle and the common pulmonary artery. The latter was replaced nine years later by a homograft valve inserted in a Hemashield conduit (Boston Scientific, Natick, MA, USA).
At the beginning of the procedure, rotational angiography (lateral to lateral) was performed (Figure 5) with breath holding and rapid pacing. The three-dimensional images clearly showed a significant stenosis in the mid-right pulmonary artery and at the distal anastomosis of the conduit on the common pulmonary artery (Table 2).
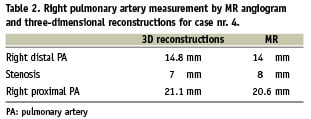
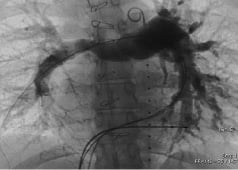
Figure 5. Still frame from the rapid pacing rotational angiography. This image shows the right pulmonary artery with contrast media injected through a MULTI-TRACK (NuMED) catheter positioned in the common pulmonary artery.
The mid-right pulmonary artery stenosis was dilated and stented with a Palmaz P3110 stent (Cordis, Johnson & Johnson, Miami Lakes, FL, USA) mounted on a BIB 16 mm x 3 cm balloon (Numed, Hopkinton, NY, USA ). The anastomotic stenosis was treated with a Palmaz P4014 stent (Cordis, Johnson & Johnson, Miami Lakes, FL, USA) on a BIB 18 mm x 4.5 cm balloon. Both interventions were performed using an optimal angiographic incidence selected from the volume rendering. Final angiographic result (rotational angiography and three-dimensional reconstructions) was excellent. There was normalisation of the right lung perfusion at nuclear test (52% of the lung perfusion).
In this case, Innova three-dimensional multi-planar reconstruction depicted well the tortuosity of the stenotic segments. Volume renderings were studied and rotated in any position to better appreciate the lesions and optimise the working views (Figure 6).
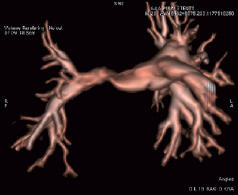
Figure 6. Volume rendering before right pulmonary artery stenting in a right anterior oblique 19° projection.
Discussion
To our knowledge, this is the first report of the feasibility of an imaging protocol providing three-dimensional reconstructions obtained directly in the catheterisation laboratory from rotational, single-plane acquisitions performed with rapid pacing and breath holding.
A large format, digital, flat-panel angiographic system was used in this study. This technology had enhanced image quality compared to conventional image intensifier system4. Software which allows three-dimensional reconstructions from rotational single plane acquisitions are nowadays often available in the catheterisation laboratories. Three-dimensional, multiplanar visualisations allow true orthogonal measurements – without a calibrating catheter – of vascular diameter during the procedure. Volume renderings help to appreciate the lesions and to optimise the working views as they can be rotated and studied in multiple views. In the presence of multi-segment lesions, often associated with pulmonary artery stenosis, one acquisition allows us to select optimal angiographic incidences for the different lesions, thus decreasing the time spent for the procedure and the amount of contrast agent used.
Bench tests of rotational angiography for three-dimensional acquisitions in a larger 40 cm field of view suggests that the radiation dose-per-frame (84.1 µGy/frame) is comparable to the standard two-dimensional acquisition in a 20 cm field-of-view (91.2 µGy/frame). Therefore, to image complex structures, which often require two or more standard two-dimensional acquisitions (70-100 frames per acquisition), this novel imaging technique may offer a substantial reduction in total radiation dose, as complete acquisition for three-dimensional reconstructions requires a total of 150 frames.
Rotational angiography with three-dimensional reconstructions is traditionally reserved to peripheral imaging such as in neurovascular radiology5-7 to facilitate better perception of the vasculature in three-dimensions. Concerning cardiac applications, rotational angiography with three-dimensional reconstructions was described in 2001 to solve an anatomical enigma in a case of repaired aortic coarctation8. More recently, it has been used by Orlov9 et al in the electrophysiology laboratory to image the left atrium, pulmonary veins and the oesophagus during ablation procedures. There is also a proposal to image the coronary vasculature using rotational angiography. However, all these imaging were obtained without rapid pacing.
The objective of rapid pacing is to reduce the motion artefacts and decrease the contrast volume. By decreasing the cardiac output, rapid pacing allows a slowed wash-out of contrast media during the five seconds rotation over 200 degrees, which results in better opacification of the vascular structures and hence enhanced image quality. Rapid pacing probably has the greatest utility in patients with a normal ejection fraction, as in our series.
In our protocol, there is no pre-specified objective concerning the drop of blood pressure. A real cardiac standstill in our setting is considered less important than rapid pacing used for device delivery such as in TAVR. This is one reason we have chosen to pace only at 180 bpm instead of 220 bpm, which is the rapid pacing rate commonly used in TAVR. Another reason we pace at a slower rate is the concern that rapid pacing may provoke potentially harmful ventricular arrhythmias, especially in the setting of patients with significant structural heart disease.
In the Webb et al series of 40 consecutive patients treated by TAVR for severe aortic stenosis, ventricular fibrillation complicated rapid pacing in one patient and was responsive to a single defibrillation1. In two series of rapid pacing used to facilitate precise stent deployment during percutaneous coronary interventions10,11, there were no adverse events. Despite the fact that rapid pacing seems to be safe, immediate access to defibrillation is always required.
Other techniques have been described to obtain a reduction in cardiac output, such as induced ventricular fibrillation or pharmacological methods2,3. Adenosine-induced ventricular standstill has been used during congenital cardiac catheter interventions and endoluminal aortic stent graft implantation. However, adenosine metabolism is very rapid and its effect is dependent on the dose and route of administration. Therefore, the time of onset is difficult to predict as is the duration of ventricular standstill, whereas rapid pacing is a reproducible method of rapid and reversible reduction of transvalvular and transvascular flow.
In conclusion, rotational angiography with rapid pacing is safe and effective for obtaining real-time, three-dimensional reconstruction imaging in the cardiac catheterisation laboratory. The advantages of this innovative imaging technique include rapid image acquisition and processing, with a precise definition of complex structures while reducing the amount of contrast media used. It is therefore a good tool to guide percutaneous interventions, especially for structural heart disease interventions.
Online data supplement
Video 1. Rapid pacing rotational angiography from right anterior oblique 100º to left anterior oblique 90º.
Video 2a. Maximum Intensity Projection (MIP) pre-stent implantation.
Video 2b. Volume rendering pre-stent implantation. This reconstruction helped to appreciate the lesions and optimise the working views.
Video 3. Rapid pacing, rotational angiography post-covered-stent implantation from right anterior oblique 100º to left anterior oblique 90º.

