
Abstract
Aims: Complex single ventricle topography, changes in vessel geometry after surgical steps and subsequent stenoses are difficult to visualise with biplane conventional angiography (CA). This study aimed to investigate the additional value of three-dimensional rotational angiography (3DRA) compared to CA for diagnostic and interventional purposes in children with univentricular hearts.
Methods and results: Demographic data, clinical data and catheterisation details of both imaging techniques were collected retrospectively. Image quality, interventional success and the additional value of 3DRA were reviewed and scored. Between January 2003 and March 2017, 140 patients underwent 183 CAs and 107 3DRAs. 3DRA image quality was superior to CA with fewer diagnostic angiographies performed (p<0.001). Intervention rate (p<0.001) and interventional success (p=0.03) were higher with 3DRA, while complication rates were similar. Mean radiation was lower in the 3DRA group, reaching significance pre-PCPC. 3DRA was considered of additional value in imaging of cardiovascular anatomy, collaterals, stenoses, and vessel-vessel and vessel-bronchi interactions.
Conclusions: In univentricular hearts, 3DRA provides superior image quality when compared to CA. Furthermore, 3DRA is performed with fewer diagnostic angiographies, less radiation and higher interventional success.
Abbreviations
Ao: aorta
CA: conventional angiography
DAP: dose area product
DKS: Damus-Kaye-Stansel
PA: pulmonary arteries
PCPC: partial cavopulmonary connection
3D: three-dimensional
3DRA: three-dimensional rotational angiography
TCPC: total cavopulmonary connection
Introduction
Univentricular hearts comprise about 10% of all congenital heart defects1. Children with a univentricular heart are palliated in three stages: the Norwood or Damus-Kaye-Stansel (DKS) procedure with a systemic-pulmonary artery shunt, the Glenn procedure (partial cavopulmonary connection [PCPC]), and the Fontan procedure (total cavopulmonary connection [TCPC]), respectively2. Topography in single ventricle physiology is complex due to the abnormal position, size and haemodynamics of cardiovascular structures. Besides, the geometry of vessels and interactions with other chest structures change tremendously with each surgical step, often resulting in vessel stenosis difficult to detect by biplane conventional angiography (CA). Therefore, detailed three-dimensional (3D) imaging may provide essential additional preoperative information.
Three-dimensional rotational angiography (3DRA) provides real-time 3D images and is suitable for displaying complex cardiovascular anatomy3,4. In addition, the acquired images can be used as a roadmap to guide subsequent percutaneous interventions5. Most studies on 3DRA in congenital heart disease describe data of both children and adults, in small sample sizes and with a broad spectrum of heart defects. These reports mainly focus on radiation dose, interventional applications and technical improvement6-9.
This study investigated the diagnostic accuracy and additional value of 3DRA compared to CA for diagnostic and interventional purposes in children with univentricular hearts. In addition, 3DRA technical settings were analysed to design a 3DRA imaging protocol.
Methods
STUDY DESIGN AND POPULATION
A retrospective study was performed under a waiver obtained from the institutional review board. Children younger than 18 years with univentricular hearts receiving catheterisation between January 2003 and March 2017 in the Wilhelmina Children’s Hospital, Utrecht, were included. A univentricular heart was defined as a cardiac condition requiring the Fontan circulation as a palliation. Catheterisations were included if their aim was to display anatomy before surgery: pre-PCPC or pre-TCPC, or when interventions of the pulmonary arteries (PA) and/or aorta (Ao) were performed. All 3DRAs performed in children with univentricular hearts were analysed to design a 3DRA imaging protocol for the three different Fontan stages.
IMAGE ACQUISITION
All catheterisations were performed under general anaesthesia by the same team of clinicians. 3DRA became available from September 2011 onwards (Artis zee biplane; Siemens, Erlangen, Germany). Prior to 3DRA, the Siemens BICOR top biplane interventional cardio angiography system with image intensifiers was used. Fluoroscopy frame rates were 6.25-12.5/second for fluoroscopy and 12.5-25/second for cine angiography. Frame rates for the Artis zee biplane were 10 or 4/second for fluoroscopy, 60 or 30/second for 3DRA and 15 or 10/second for cine angiography.
Siemens’ syngo DynaCT cardiac software and Leonardo workstation were used to reconstruct the 3D model automatically after obtaining the rotational angiographic images and to post-process for different purposes with different characteristics (e.g., opacity and low-/high-density threshold). Data sets could be combined, planes clipped to delineate stenosis (fly-through) and vessels viewed endoscopically (Moving image 1). In some cases, an empty 3DRA was made (without contrast) for subsequent uploading of a recently made 3DRA to use as a roadmap to guide intervention.
DATA COLLECTION
Demographic data, clinical data and catheterisation details of all included procedures were collected or scored from patient files, catheterisation databases (FileMaker Pro 11; FileMaker Inc., Santa Clara, CA, USA) and image databases (Philips Xcelera R4.1; Philips Medical Software, Eindhoven, the Netherlands; and OsiriX MD 2.6; Pixmeo SARL, Bernex, Switzerland). Numbers of angiographies (A- and B-plane) were scored as diagnostic (all series with contrast for visualisation of anatomy) or interventional (all series with balloon dilation, stent implantation or embolisation with coils/plugs and contrast after intervention). Visibility of ventricle morphology, pulmonary arteries, aorta, coronary arteries, aortopulmonary shunt, caval veins and innominate veins were scored on a three-point scale to assess image quality. Good image quality was defined as full diagnostic image quality with clear delineation of cardiovascular structures, moderate image quality as providing an estimated delineation, and poor image quality as providing no delineation. In case of doubtful image quality, an interventional cardiologist independently reassessed image quality.
The additional value of the included 3DRAs was evaluated by two interventional paediatric cardiologists, scored on a scale of 1 to 3 (1: 3DRA of no additional value for understanding anatomy or guiding interventions, 2: 3DRA of some additional value, and 3: 3DRA of high additional value in understanding anatomy or guiding intervention).
All interventions performed were analysed and scored on type of intervention and successfulness. Success was defined as significantly improved pressure gradients and/or vascular diameter for balloon or stent interventions, and occlusion of collaterals in coil interventions. Moderate success was defined as limited improvement of pressure gradients or vascular diameter in balloon or stent interventions. An intervention was not successful if there was no improvement of pressure gradients or vascular diameter (balloon or stent intervention), or when a collateral was still open. Complications were collected in line with current guidelines10.
DATA REARRANGEMENT: DICHOTOMISATION
After data collection, sample sizes for poor and moderate image quality and moderate and no interventional success were small. Therefore, dichotomisation was applied to increase power for statistical analysis. For image quality, the good category remained and the moderate and poor categories were aggregated to the “not good” category. The successful category remained for interventions, but the moderate scored interventions were added to the not successful category.
STATISTICAL ANALYSIS
Data were analysed using IBM SPSS Statistics software, Version 21 (IBM Corp., Armonk, NY, USA). Summaries of variables are presented as numbers, percentages of total, or mean with standard deviation (SD). Variables of 3DRA performance are summarised as a median with range (min-max). The generalised estimating equation was used to analyse differences in radiation and number of angiographies. The independent t-test or chi-squared/Fisher’s exact test was used to determine differences in catheterisation characteristics, diagnostic accuracy, interventions, complications and amount of contrast used. A p-value <0.05 was considered significant.
Results
3D ROTATIONAL ANGIOGRAPHY VERSUS CONVENTIONAL ANGIOGRAPHY
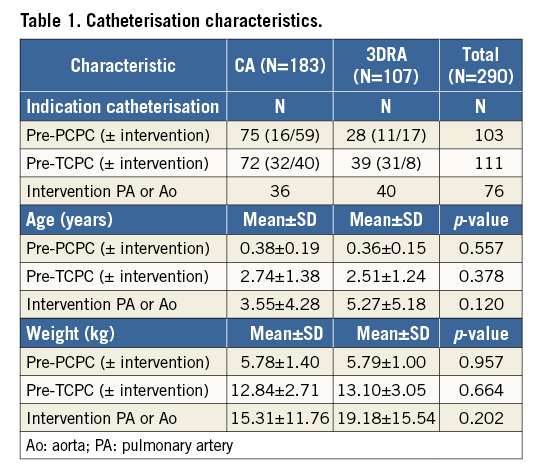
A total of 290 catheterisations were performed in 140 patients (60.7% male, mean 2.07/patient; range 1-5), comprising 183 CAs (63.1%) and 107 3DRAs (36.9%, of which four were empty runs). Extended details on patient diagnosis and surgical history are shown in Supplementary Table 1. Of all catheterisations, 124 were primarily diagnostic (pre-PCPC or pre-TCPC), 76 were primarily interventional (intervention on pulmonary arteries and/or aorta) and 90 were intended for diagnostic purposes but resulted in intervention as well (pre-PCPC or pre-TCPC with intervention). There were no statistically significant differences between the subgroups (Table 1).
Cardiovascular anatomy was displayed superiorly with 3DRA compared to CA (Table 2). During 166 interventional catheterisations 309 interventions were performed. In the CA group, 145 interventions were performed in 84 catheterisations (45.9% of included CA procedures) and, in the 3D group, 164 interventions in 82 catheterisations (76.6% of included 3D procedures) (p<0.001). Interventions included 70 balloon dilations and 78 stent interventions for coarctations, pulmonary artery stenosis and shunt stenosis (Supplementary Table 2). In addition, 148 collaterals were closed and 14 other interventions were performed. There was no difference in distribution of type of intervention between the two groups (data not shown). The mean number of interventions per catheterisation was similar: 1.73±1.00 (range 1-5) for CA and 2.00±1.13 (range 1-7) for 3DRA, p=0.100. Interventional success rates were higher in 3DRA-guided interventions: 94.5% (n=155) when compared to CA 87.6% (n=127), p=0.03.
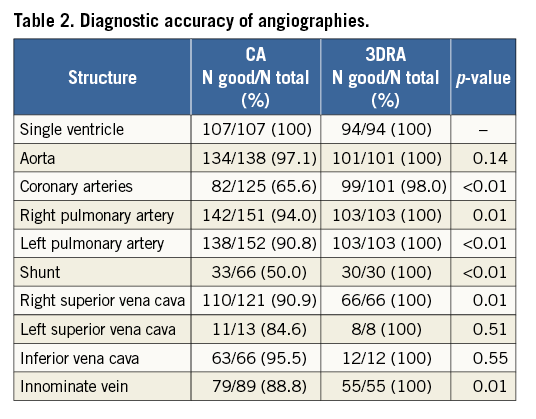
Procedural complications occurred in 12 CAs (6.6%) and seven 3DRAs (6.5%), p=0.996. These complications were not related to 3DRA as a technique (e.g., no arrhythmias due to rapid pacing or pulmonary hypertension occurred), but to the catheterisation itself. Interventional complications occurred in 12 CA and 10 3DRA procedures. Compared to the total number of interventions (145 conventional and 164 3D-guided), these numbers of complications did not differ: 8.3% and 6.1%, respectively, p=0.457.
The difference in number of diagnostic angiographies was analysed for all diagnostic catheterisations. There were significantly fewer diagnostic angiographies, A- and B-plane, performed in the 3DRA group compared to the CA group: 4.72±2.80 versus 6.37±3.05 (p<0.001) and 2.67±1.97 versus 4.99±2.40 (p<0.001), respectively.
Total radiation dose area product (DAP) was analysed for five different subgroups based on reason for catheterisation: pre-PCPC, pre-TCPC, pre-PCPC with intervention, pre-TCPC with intervention and sole interventional catheterisations (Table 3). Mean radiation was significantly lower for 3DRA in the pre-PCPC subgroup (p<0.001). In the other groups, radiation was lower with 3DRA, but did not reach significance (p-values 0.382, 0.584, 0.359 and 0.683, respectively) with a similar number of interventions (Table 3).
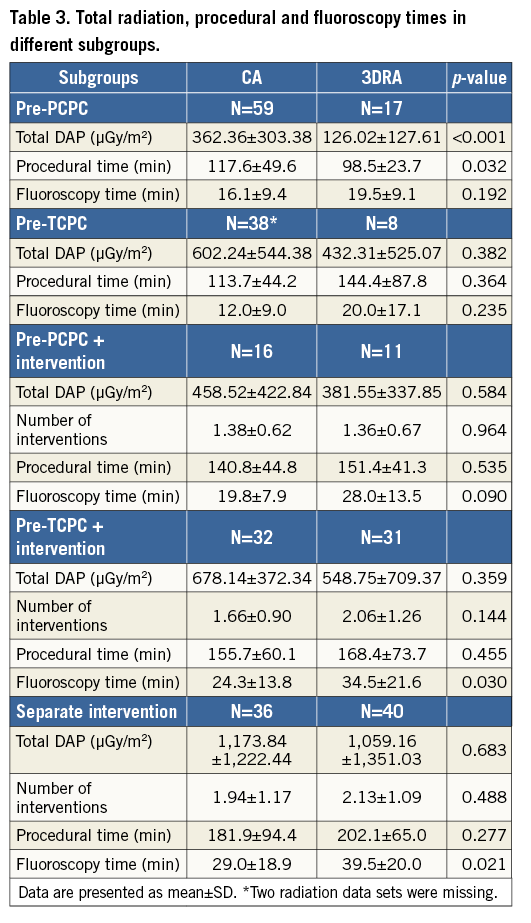
The procedural time was significantly shorter in the 3DRA pre-PCPC group (p=0.032) and did not differ in the other subgroups. Fluoroscopy times were significantly longer for 3DRA in the pre-TCPC with intervention (p=0.030) and interventional groups (p=0.021).
There was no difference in the amount of pure contrast used for diagnostic imaging between CA and 3DRA: mean 4.38±1.76 ml/kg in CA and 4.08±1.46 ml/kg in 3DRA, respectively (p=0.211).
ADDITIONAL VALUE OF 3DRA
The mean score for 3DRA additional value was 2.40, with 45 3DRAs scoring three points and 54 3DRAs scoring two points. A score of one was given four times as additional conventional angiographies were performed or no additional findings were found.
Collaterals were displayed in 70 of the 103 3DRAs (68.0%) (Figure 1A-Figure 1C). Interactions between vessels (n=47, 45.6%) and between bronchi and vessels (n=17, 16.5%) were adequately visualised (Figure 1D-Figure 1F, Moving image 2). The use of 3D reconstructions and angulations was considered of additional value in vessel course, vessel-vessel and vessel-bronchi interactions and vessel stenosis in 48 of 103 3DRAs (46.6%) (Figure 2A, Figure 2B). In 19 cases (18.4%), 3DRA showed significant additional findings including arteria lusoria, interarterial course of a coronary artery, extracardiac tunnel morphology, inflow and outflow tract morphology including outflow tract aneurysm after Sano shunt.
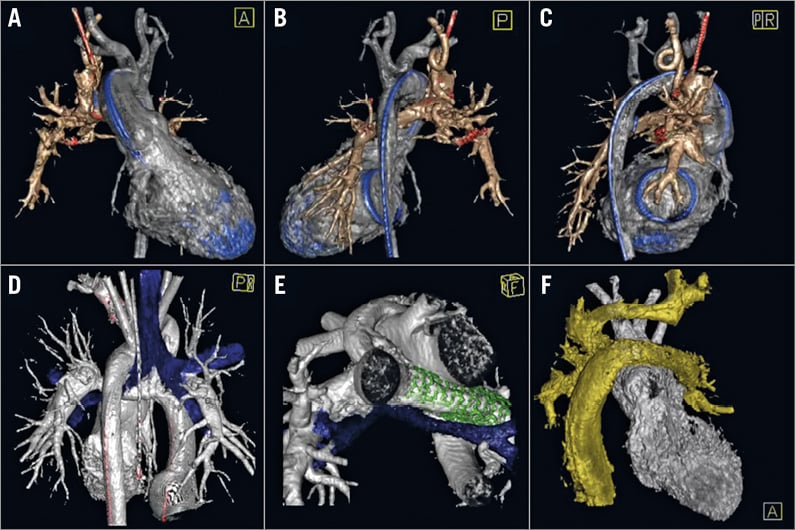
Figure 1. Collateral, vessel-bronchus and vessel-vessel interaction visualised by 3DRA. Major aortopulmonary collateral artery visualised behind the superior caval vein in a two-year-old girl with a PCPC and mechanic mitral valve, from anterior (A), posterior (B) and right-posterior (C) views. Vessel-bronchus relationship in an 11-year-old boy with TCPC prior to (D) and after (E) left pulmonary artery stenting from posterior and caudal view, respectively. Vessel-vessel relationship of left pulmonary artery compression on DKS in a four-year-old girl with a Lecompte, reconstructed arch, LPA stents and TCPC palliation for tricuspid atresia, transposition of the great arteries and hypoplastic aortic arch/CoAo (F). A) - C) Silver: ventricle and aorta; bronze: PCPC; blue: catheter and mechanic valve. D) & E) Silver: ventricle, aorta and TCPC, blue: airway; green: stent. F) Silver: ventricle, aorta/DKS; gold: TCPC, innominate vein, left jugular vein.
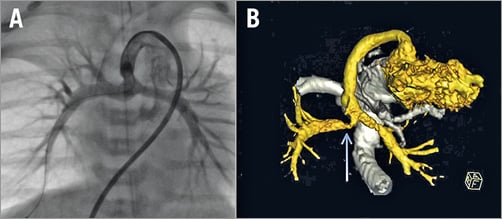
Figure 2. Right pulmonary artery stenosis in a 10-day-old boy with hypoplastic left heart syndrome and Sano shunt. Conventional angiography showing the shunt and pulmonary arteries without stenosis (A). 3D reconstruction in semi-caudal orientation showing a severe right pulmonary artery stenosis (B) (blue arrow).
Of the 107 3DRAs, 82 were interventional (76.6%). 3DRA reconstructions were used as roadmap guidance, which facilitated sheath and device positioning (Figure 3A). In some of the roadmap-guided interventions an anatomy shift occurred as a result of vessel deformation by stiff sheaths, wires or device positioning (Figure 3B). In 15 cases (18.3%), additional diagnostic information obtained with 3DRA (e.g., vessel-vessel and vessel-bronchi relationships/interactions) led to a change in interventional strategy.
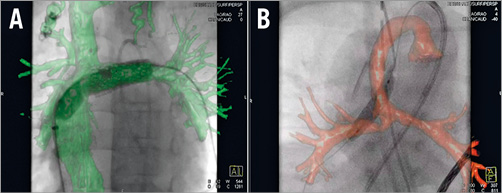
Figure 3. Roadmap-guided intervention. 3D reconstruction-guided intervention without anatomy shift in a four-year-old girl with tricuspid atresia, TCPC and left pulmonary artery stent stenosis (A). 3D reconstruction-guided intervention with anatomy shift in a 10-day-old boy with hypoplastic left heart syndrome, Sano shunt and right pulmonary artery stenosis (B).
3DRA IMAGING PROTOCOL
Between 2011 and 2017, 101 patients with univentricular hearts received a total of 149 3DRA catheterisations (mean 1.50 per patient; range 1-4). Throughout the years, radiation exposure decreased as well as procedural and fluoroscopy times (Table 4). After our own in vitro benchmark testing with different settings, the rotational frame rate was lowered from 60 to 30 frames/second from 2013 onwards without any significant loss of detail in structures (data not shown). With a 50% frame reduction and craniocaudal collimation applied to the region of interest, a 50% reduction in 3DRA radiation was achieved. In addition, the fluoroscopy and angiography frame rates were reduced from 10 to 4 and 15 to 10 per second, respectively.
Technical settings on rapid pacing and contrast injections were used to design a 3DRA workflow and imaging protocol (Supplementary Figure 1). Optimal pacing frequency is obtained by increasing frequency from 160 to 230 beats per minute upwards until a 50% reduction of systolic arterial pressure is achieved. Contrast is diluted with saline to 67% and administered simultaneously at multiple locations with a power injector (high flow site) and as many syringes as necessary proximal to the region or cavity of interest to create a depot and prevent washout artefacts. Our experience led to an empirical relation between body weight and contrast flow in ml/s for the power injector (Supplementary Figure 1). The total amount of diluted contrast results by multiplication of the contrast flow with the seconds necessary to keep the contrast saturation stable at the cavity of interest (5 seconds plus X-ray delay). The total amount of diluted contrast for manual injections is a percentage of the contrast amount given by power injection and is divided over the different injection sites for PCPC or TCPC (Supplementary Figure 1).
Discussion
Since its introduction in our centre in 2011, 3DRA has become the standard imaging method for the diagnostic evaluation and percutaneous treatment of children with univentricular hearts stage I to III. This is the first study to evaluate the additional value of 3DRA in the diagnostic evaluation and percutaneous treatment of children with univentricular hearts when compared to conventional angiography. Our results outline that 3DRA is superior to CA regarding image quality, radiation dosages and interventional success in a large population of patients.
3DRA displayed all cardiovascular and surrounding structures with excellent quality in one run in contrast to CA. The latter provided two-dimensional images of vasculature with fairly good quality, with multiple angiographies necessary to visualise each substrate outside its topographic context. The optimal image quality of 3DRA is achieved by the use of a protocol. Rapid pacing decreases the cardiac output resulting in higher contrast density and prolonged contrast transition time or contrast stasis when compared to CA6,11,12. Timing and location of contrast injection is essential. The combination of contrast pre-filling by X-ray delay, multiple location contrast injection with the power injector and simultaneous manual injections provides a constant contrast supply and contrast density immediately before and during the entire image acquisition. This enables dynamic evaluation when the rotational angiography information is studied and high-resolution static information of cardiovascular structures after 3D post-processing. Typical limitations of CA such as suboptimal angulation, over-projection errors and the need of multiple sequential selective angiographies can be overcome with 3DRA. In the end, this results in high-quality 2D rotational and 3D reconstructed images that are more precise and better in displaying the cardiovascular anatomy and its surrounding tissues compared to CA, which can also be printed as a 3D model (Moving image 3).
With implementation of 3DRA it is important to evaluate radiation and to compare it to CA. Total DAP was significantly lower for 3DRA in the pre-PCPC group. In the other subgroups, radiation was lower than with CA but did not reach significance. It must be stated, however, that all 3DRAs were performed with flat panel detectors whereas the majority of CAs were performed with the older BICOR system. This could be a small additional factor explaining the observed reduction in DAP after implementation of 3DRA. Improvement of the 3DRA workflow with lower frame rate, collimation and optimised timing led, however, to a more dramatic dose reduction. These improvements were the results of our learning curve and also of a change in mind set and strategy as 3DRA made us aware of radiation (detailed X-ray data). Fewer additional angiographies are performed due to the use of a roadmap for interventions and the possibility of rotating the reconstruction in search of the best angulation for diagnosis. The mean DAP of one 3DRA run decreased from 388.76 μGy/m2 in 2011 to 179.20 μGy/m2 in 2016. This mean 3DRA DAP is equal to 1 to 1.5 biplane angiographies. Berman et al reported a mean 3DRA run DAP of 558.72 μGy/m2 in children with bidirectional Glenn or Fontan, with a mean age and weight of 5.85 years and 23 kilograms, respectively3. In our cohort of children >2.25 years, mean 3DRA run DAP, age and weight were 391.42 μGy/m2, 6.26 years and 22.9 kilograms, respectively. Corredoira and colleagues describe median 3DRA run DAPs per age category. Below the age of one year a median 3DRA run DAP and weight of 63.9 μGy/m2 and 5.6 kilograms for diagnostic and 56.9 μGy/m2 and 5.9 kilograms for interventional purposes was reported9. Median 3DRA DAPs and weights for diagnostic and interventional catheterisations in our cohort were 55.4 μGy/m2 and 5.7 kilograms and 53.3 μGy/m2 and 6.0 kilograms, respectively. Corredoira et al adjusted the DAPs for attenuation of radiation by table and mattress resulting in lower DAPs. Our results are favourable when compared to these studies. Furthermore, with an increased experience in 3DRA, the need for additional CAs decreased, leading to a further reduction in radiation.
The number of additional diagnostic angiographies was significantly lower in the 3DRA group. Complete cardiac morphology was displayed with one 3DRA run, which made repetitive selective CAs unnecessary. The procedural time was significantly shorter in the 3DRA pre-PCPC group (p=0.032). On the other hand, fluoroscopy times were significantly longer for 3DRA in the pre-TCPC with intervention (p=0.030) and interventional groups (p=0.021). These longer fluoroscopy times are probably due to more complex interventions performed, but more importantly did not result in higher total DAPs in these patients. Compared to another study describing both procedural time and fluoroscopy time in congenital heart disease, our results are similar. Haddad and colleagues report a median procedural time for 3DRA of 140 minutes (IQR 110-207) whereas ours was 136 minutes (IQR 105-210; range 30-420). Their median fluoroscopy time was 30.8 minutes (IQR 17-55) compared to 26.9 minutes (IQR 15.6-40.5; range 1.2-120.2) in our population13. The amount of contrast used did not differ between the 3DRA and CA groups. More contrast was used for one all-in-one 3DRA run compared to one CA, but multiple CAs were required to evaluate the single ventricle physiology.
Interventional success was higher in the 3DRA group: 94.5% versus 87.6% with CA (p=0.03). This could be the result of the increased experience of the catheterisation team in performing complex interventions in single ventricle patients. In our opinion, understanding of cardiac anatomy is incomplete when based solely on CA and may lead to undertreatment in this patient group. The increased experience of the team, in combination with the increased awareness of morphological substrates provided with 3DRA, lowered the threshold of performing interventions in this patient group. Furthermore, it is our opinion that lesions (e.g., vessel stenosis or relevant collaterals) should be tackled as soon as possible to improve Fontan function in the long term. This is reflected by an increased intervention rate in the 3DRA group: 82/107 versus 84/183 in the CA group (p<0.001), with similar distribution of type of intervention.
Complication rates were similar in the 3DRA and CA groups. Procedural complication rates were 6.5% in the 3DRA and 6.6% in the CA group and interventional complication rates were 6.1% and 8.3%, respectively. These numbers are lower than those reported in the literature: 10% for diagnostic and 20% for interventional cases10. No complications could be directly related to the 3DRA technique, showing that 3DRA can be safely used in stage I-III palliation of children with univentricular hearts. This is demonstrated by our smallest patient weighing 2.2 kilograms suffering from an acute postoperative shunt stenosis.
Univentricular hearts have a complex anatomy with interventional substrates varying widely. Vessel-vessel and vessel-airway relationships cannot be demonstrated by CA14,15. This can lead to iatrogenic compression of coronary arteries, pulmonary veins or bronchi. 3DRA, being computed tomography angiography (CTA) at the catheterisation laboratory, overcomes these potential complications by visualisation of the entire topography in unlimited angulations. In a few cases, the left pulmonary artery (LPA) was not stented as an “empty” 3DRA with balloon interrogation with the targeted stent diameter showed airway compression. Also, low-flow venovenous as well as high-flow aortopulmonary collaterals are easily displayed by 3DRA without the need for additional superselective CAs. Access and closure are simplified with the use of 3DRA roadmaps. Furthermore, roadmapping in general facilitates sheath and device positioning without the need for repetitive CAs. However, 3D static roadmap intervention guidance can be misleading, as tissue shift may occur due to respiration, patient movement or with the use of stiff guidewires or sheaths. These anatomy shifts occur in one third of patients and are just as common in all three stages. In some cases, this problem can be tackled by adjusting the overlay with an additional angiography, but unfortunately correction is not always possible. If an anatomy shift disturbs the planned intervention, the overlay will not be used.
Since 3DRA was first introduced in our institution, both procedural time and fluoroscopy time decreased as the interventional cardiologists became faster and more confident in performing the 3DRA-based procedure. In the vast majority of cases, the operators found that 3DRA was of high additional value in understanding anatomy or guiding intervention. This experience was translated to a 3DRA imaging protocol for children with univentricular hearts (Supplementary Figure 1).
Limitations
Our study is limited by its retrospective, single-centre design. Our results cannot be generalised to children with other congenital heart defects. In the future, prospective studies on different forms of congenital heart disease should be performed at multiple institutions.
Conclusions
In univentricular hearts, three-dimensional rotational angiography provides superior image quality when compared to conventional angiography. Complex morphology of stenoses, and vessel-vessel and vessel-airway interactions are visualised by 3DRA. Furthermore, 3DRA is performed with fewer diagnostic angiographies, less radiation and higher interventional success. Therefore, 3DRA should be the preferred technique for diagnostic imaging and percutaneous treatment of children with univentricular hearts in any of the Fontan stages when available.
| Impact on daily practice Three-dimensional rotational angiography (3DRA) provides superior imaging of complex single ventricle topography compared to conventional angiography (CA), including complex morphology of stenoses, and vessel-vessel and vessel-bronchi interactions. The procedure is performed with less radiation and increased interventional success. These beneficial results in combination with a ready-to-use imaging protocol should stimulate interventional cardiologists to use 3DRA for diagnostic evaluation and percutaneous treatment of children with univentricular hearts. |
Conflict of interest statement
G. Krings is a member of the Siemens Advisory Board and a consultant for Edwards Lifesciences. The other authors have no conflicts of interest to declare.
Supplementary data
Supplementary Table 1. Diagnosis and surgical details pre-catheterisation.
Supplementary Table 2. Interventions performed per stage.
Supplementary Figure 1. 3DRA run workflow and image acquisition settings.
Moving image 1. Pre- and post-interventional 3D reconstruction of a 14-year-old girl with tricuspid atresia, pulmonary atresia and hypoplastic right ventricle palliated with intracardiac TCPC; status post tunnel stent and left pulmonary artery (LPA) stent.
Moving image 2. Workflow of 3D reconstruction, including airway, from a 3DRA data set of a two-year-old girl with partial cavopulmonary connection and mechanic mitral valve.
Moving image 3. Workflow of a 3DRA DICOM data set to 3D print of a 10-day-old boy with Sano shunt and severe right pulmonary artery stenosis.
To read the full content of this article, please download the PDF.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1.
Moving image 2.
Moving image 3.

