
Abstract
Aims: Left pulmonary artery (LPA) stenosis is common in patients with cavopulmonary connections. Stent implantation is the treatment of choice but may be complicated or contraindicated by left main bronchus (LMB) compression due to limited retro-aortic space after a Damus-Kaye-Stansel (DKS) or Norwood operation. This study describes a novel double balloon technique of LPA stenting in patients at risk of LMB compression.
Methods and results: A cohort study was performed in 11 patients who underwent LPA stenting with an oval stent technique between 2015 and 2018. Retro-aortic anatomy was evaluated periprocedurally by three-dimensional rotational angiography (3DRA). Pre-existing LMB compression was demonstrated by 3DRA in seven out of eight patients who had undergone previous LPA stenting and in one patient without stenting. Primary ovalisation with immediate stent implantation on double balloons was performed in one patient. Ten patients had secondary ovalisation with single balloon stent implantation followed by the double balloon technique for ovalisation. The procedures were successful in all patients and guaranteed LMB patency without increasing pre-existing compression.
Conclusions: The 3DRA-guided oval stent technique with double balloon inflation is successful in treating LPA stenosis after a DKS or Norwood operation in patients at risk of bronchial compression, guaranteeing LMB patency without increasing pre-existing compression.
Introduction
Around 10% of all congenital heart defects (CHD) are eventually palliated with a total cavopulmonary connection (TCPC)1. Left pulmonary artery (LPA) stenosis is common after this palliation and frequently requires stent treatment2. The retro-aortic space, including the LPA and left main bronchus (LMB) between the neo-aorta and descending aorta, is limited after the Norwood or Damus-Kaye-Stansel (DKS) procedure3,4,5. Advanced periprocedural imaging with three-dimensional rotational angiography (3DRA) has increased the awareness and understanding of these close vessel-vessel and vessel-airway interactions6,7,8. Compression of the LMB is increasingly seen as a complication in or contraindication for LPA stenting in these patients9. Several techniques have been suggested to prevent this complication10,11. However, to date no real solution has emerged. LMB compression could be avoided with a vertical oval stent geometry. We developed a novel 3DRA-guided stent technique to perform oval LPA stenting safely in single ventricle patients at risk of or with pre-existing LMB compression. The objective of this study is to present our current experience with this novel oval stent technique.
Methods
STUDY DESIGN
A cohort study was performed of all patients with single ventricle physiology who underwent LPA stenting with our oval stent technique. All parents gave consent for the procedure. This study was approved by our local institutional review committee and individual consent for data collection was waived.
PROCEDURE DESCRIPTION AND IMAGE ACQUISITION
Cardiac catheterisation was performed under general anaesthesia in all patients. Complete haemodynamic assessment was performed, including pulmonary wedge pressures and pressure pullback loops. 3DRA was obtained using the Artis zee system with syngo DynaCT (Siemens Healthineers, Forchheim, Germany) and our 3DRA univentricular heart protocol8. Post-processing routinely included heart, vessel-vessel and vessel-airway interactions. In addition, in the retro-aortic space, the distance between the LPA and neo-aorta, coronary arteries, LMB and descending aorta was assessed in anteroposterior, lateral and cranio-caudal orientation. If necessary, an additional “empty” 3DRA (3DRA without contrast solely to visualise airway, other stents in situ and contrast-filled balloon) was made in unstented patients using a single balloon with the target stent diameter in the LPA to evaluate future airway patency. After these diagnostics, an oval stent procedure was performed. Airway patency was evaluated post stent ovalisation by “empty” 3DRA or bronchoscopy. Pressure pullbacks were performed to assess residual gradients.
DATA COLLECTION AND PRESENTATION
Baseline characteristics and indications for cardiac catheterisation and LPA intervention were obtained. The sheaths, wires, balloons and stents used for the procedure were analysed. The different techniques used for ovalisation were summarised and evaluated. Procedural complications were noted, image quality assessed, and radiation data collected. Data are presented as frequencies or median with range.
Results
In our total population of paediatric patients with single ventricle physiology 51/204 (25.0%) underwent LPA stenting. Between January 2015 and November 2018, 11 of them (21.6%) had the oval stenting procedure. Four patients were initially palliated with a Norwood operation, six patients received a DKS procedure and one patient a central aortopulmonary shunt. Ten patients had a TCPC at the time of the procedure and one patient had a partial cavopulmonary connection (PCPC). Median age and weight at oval stenting were 11.1 years (range 2.0-17.2) and 33.0 kilograms (range 11.0-68.6), respectively. The indication for the procedure was LPA stenosis in seven patients, LMB decompression in two (patients 3 and 11), evaluation of plastic bronchitis in one (patient 9) and Fontan evaluation in one (patient 10). In patient 3, a computed tomography (CT) scan showed severe compression of the LMB due to anterior compression by a wide DKS anastomosis and non-ovalised LPA stent and posterior compression by the descending aorta. Patient 11 suffered from LMB compression (2.5 mm), visualised with 3DRA and bronchoscopy, after initial circular stent placement (10 mm diameter) of a subtotal LPA stenosis in a limited retro-aortic space (15 mm). Eight patients had previously undergone LPA stenting with Mega™ LD stents (ev3 [now Medtronic], Plymouth, MN, USA) and diameters between 8 and 15 mm.
All but one patient had an invasive gradient during LPA pullback (median gradient 2 mmHg [range 0-2]). The one patient without a gradient was the patient with LMB compression on CT. 3DRA confirmed significant LPA stenosis in the nine patients with an invasive gradient. Furthermore, 3DRA showed airway compression in eight patients caused by a previously implanted LPA stent in seven. In one patient the DKS anastomosis caused severe LPA and LMB compression. The three patients without a previous LPA stent all had severe LPA stenosis. In the patients without LMB compression, there was either close proximity of the LPA stent to the LMB or LMB compression during test balloon inflation necessitating an oval stent procedure.
Oval stent procedures
CHOICE OF MATERIALS AND POSITIONING
The anterior-posterior and superior-inferior diameters were measured. Balloons with a maximum diameter of the desired anterior-posterior diameter were selected. The superior-inferior diameter was estimated by the formula: diameter = 1/3*(diameter balloon 1 + diameter balloon 2). After selection of the desired balloons, two exchange wires were positioned deep in the LPA with a parallel course. Both balloons were positioned in the stent (Figure 1A, Figure 1B). Balloons with different diameters were used when a non-oval but trapezoid shape had to be achieved (Figure 1C, Table 1). Several double balloon techniques were used to obtain a superior-inferior balloon position: push and pull of the wires, positioning of the balloons using a long sheath for extra support, consecutive balloon inflation starting with the most superiorly located balloon and the use of a steerable sheath.
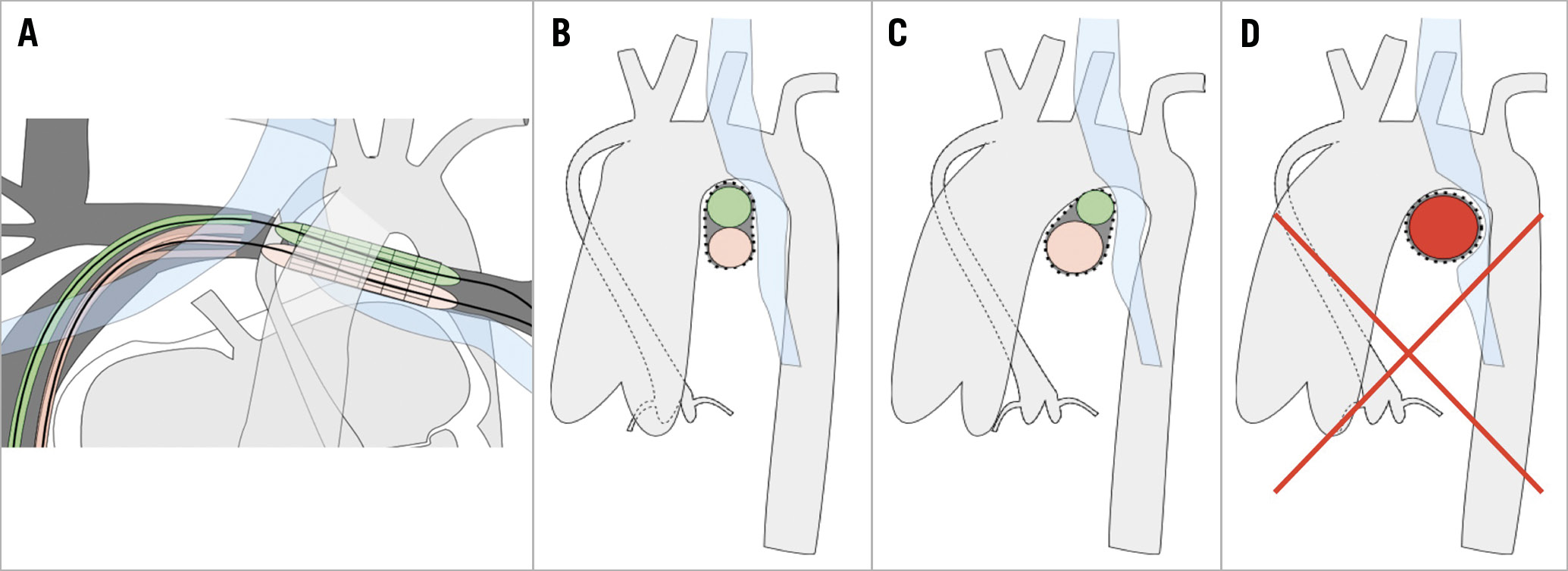
Figure 1. Schematic presentation of stent ovalisation. With the use of a steerable long sheath (pink) and conventional sheath (green), optimal superior-inferior balloon position is achieved during inflation (A-C). Lateral view to demonstrate aorta, LPA and bronchus position with symmetric double balloon stent inflation to obtain oval shape (B) and asymmetric double balloon stent inflation to obtain trapezoid shape (C). Single balloon stent inflation leading to left bronchus compression which has to be avoided (D).
PRIMARY OVAL STENT IMPLANTATION
In one patient (patient 4) with severe LPA stenosis (Figure 2A, Figure 2B), test inflation with the target balloon showed complete LMB obstruction (Figure 2C), thus circular stenting with this target size had to be avoided (Figure 1D). A smaller balloon proved unstable, indicating a high risk of stent dislocation. Primary oval stent implantation was performed with a 36 mm ev3 Mega LD stent mounted on two, 8×40 mm and 10×40 mm, POWERFLEX® balloons (Cordis [Cardinal Health], Santa Clara, CA, USA) (Figure 2D, Figure 2E). Balloons and corresponding exchange wires were marked. The stent was advanced through a 12 Fr sheath and positioned in the LPA using 3D road mapping (3DRA overlay). Initial inflation of the inferior 10 mm balloon allowed successive inflation of the 8 mm balloon in an optimal superior position.
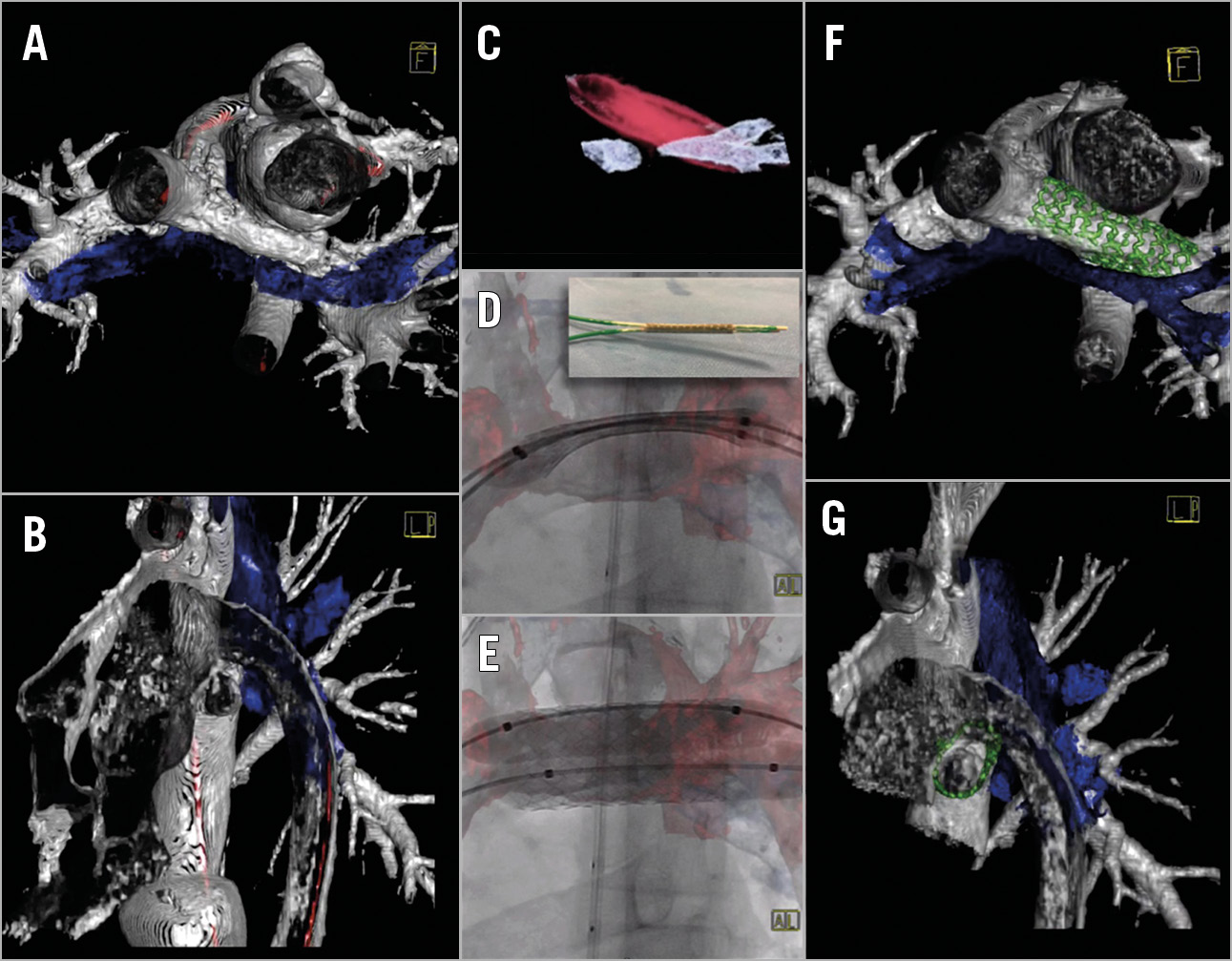
Figure 2. Primary oval stent implantation in an 11-year-old Fontan patient (patient 4) with severe LPA stenosis. 3DRA reconstruction demonstrating the LPA stenosis and vessel-airway interaction (A & B). Interrogation of the LPA with a POWERFLEX balloon 12×40 mm showed total compression of the LMB (C). An ev3 Mega LD stent 36 mm was mounted on two POWERFLEX balloons 8 and 10 mm diameter (D) which were sequentially inflated for primary oval stent implantation (D & E). 3DRA reconstruction post primary oval stent implantation shows patent LMB due to the obtained oval shape of the LPA stent (F & G). Vessels in silver; airway in blue; balloon and 3D overlay in red; stent in green.
SECONDARY OVAL STENT IMPLANTATION
IMPLANTATION AND IMMEDIATE OVALISATION
Three patients had severe untreated LPA stenosis. In two of them (patients 2 and 6), test inflation with a small single balloon showed airway patency and a clear indentation in the balloon. In these patients, stents were implanted on a single balloon used for test inflation and successively ovalised with the double balloon technique described above. In the smallest patient of the cohort (patient 2, 11 kg) with LPA stenosis after PCPC, an 8x20 mm Formula® 535 stent (Cook Medical, Bloomington, IN, USA) was re-crimped on a 4×20 mm Advance® balloon (Cook Medical) for implantation. Two coronary balloons were used for ovalisation through a single 7 Fr jugular sheath (Table 1).
OVALISATION OF STENT IN SITU
Eight patients previously underwent LPA stenting (patients 1, 3, 5, 7-11) (Table 1). The indication for ovalisation of these stents was an invasive gradient in six patients and severe airway compression with recurrent pulmonary infections in two (patients 3 and 11). Due to the close proximity of the LMB to the ascending aorta (Figure 3A, Figure 3B), anterior-posterior dilation of the stent had to be avoided. After obtaining an ideal cranial-caudal balloon position, both balloons were slowly inflated simultaneously (Figure 3C, Figure 3D). In patient 8 there was hypoplasia of the distal LPA and in patient 11 of the proximal LPA. In these patients the LPA stent was first elongated by implantation of a second stent and ovalised thereafter.
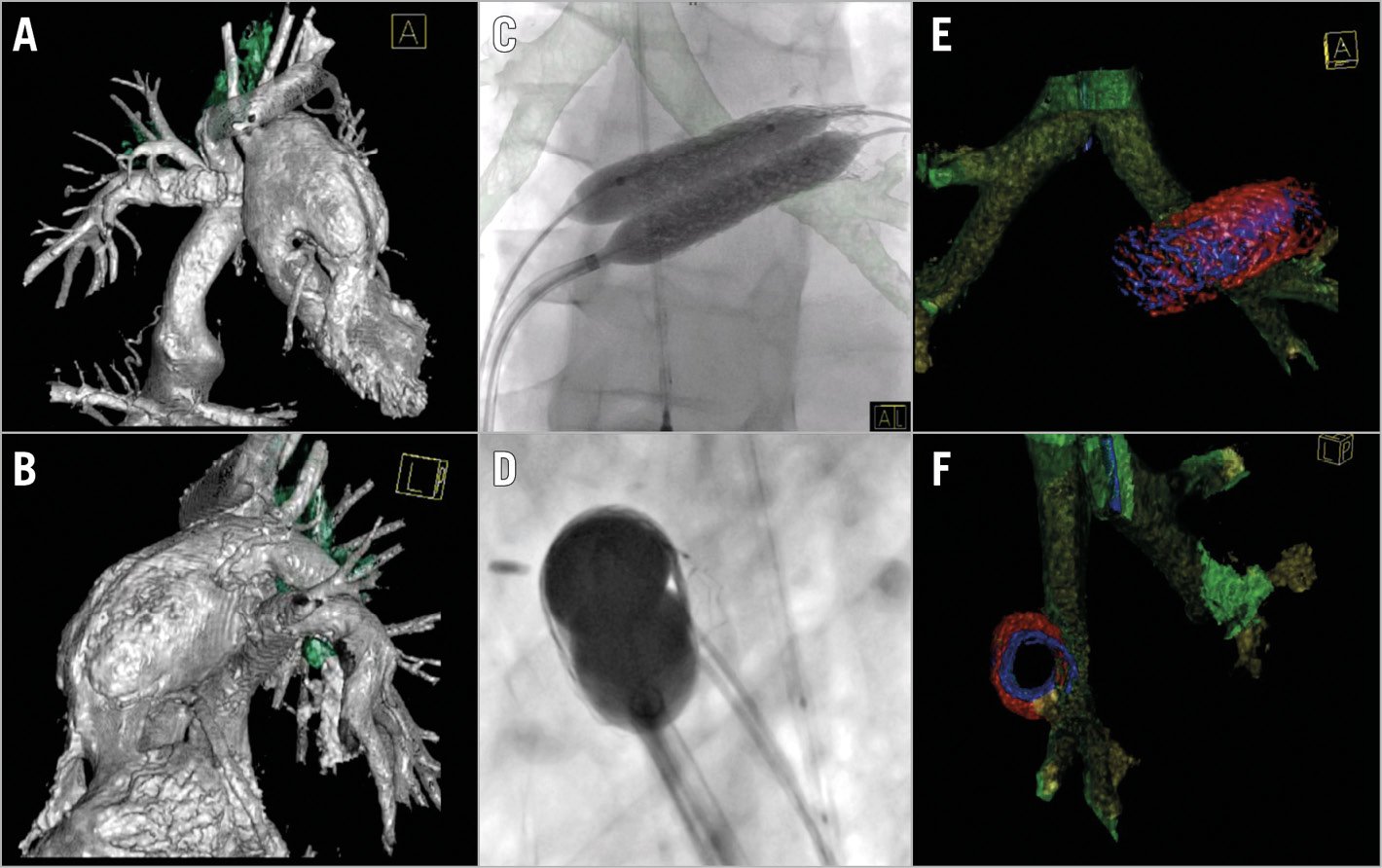
Figure 3. An eight-year-old Fontan patient with tricuspid valve atresia and hypoplastic right ventricle (patient 10) after previous LPA stent implantation. Cardiac catheterisation indicated adapting the LPA stent diameter to growth five years after implantation. 3DRA and 2D imaging showing frontal (A, C & E) and left lateral view (B, D & F) with the left main bronchus situated in between the LPA stent and the descending aorta (A & B). Oval stent technique with 3DRA overlay in frontal plane demonstrating ideal deflection of the steerable sheath to obtain superior-inferior balloon position (C & D), resulting in an oval stent shape without bronchus obstruction (E & F). Heart and vessels in silver; airway in green; initial stent dimensions in blue and dimensions after dilatation in red.
Control angiography (conventional and/or 3DRA) showed a good result in all patients with significant increment of vessel diameter and absence of contrast extravasation (Figure 2F, Figure 2G, Figure 3E, Figure 3F). There were no residual LPA gradients.
In nine patients 3DRA confirmed airway patency after the oval stent procedure. In patient 3 and patient 11, stent ovalisation as well as intrabronchial balloon dilatation (10×30 mm balloon) during the same procedure showed no immediate effect on airway diameter, despite a reduced ventral-dorsal stent diameter creating more space for the LMB in patient 11. Repeat bronchoscopy six months post ovalisation showed patency and only moderate flattening of the LMB in patient 3, with no recurrent infections during follow-up.
Two patients had a hoarse voice at discharge. Tracheoscopy by the otorhinolaryngologist confirmed left-sided vocal cord paralyses in both patients, most likely caused by left-sided phrenic nerve injury due to the LPA stent. Other than the vocal paralyses and one unsuccessful decompression of the LMB, no complications occurred.
IMAGE QUALITY AND RADIATION
3DRA image quality was excellent for vessel imaging as both the cavopulmonary connections and systemic circulation were visualised accurately in one run, with the possibility of airway reconstruction from the same data set. Visibility of airway and stent interaction was optimal in the empty 3DRA runs without intravenous contrast injection.
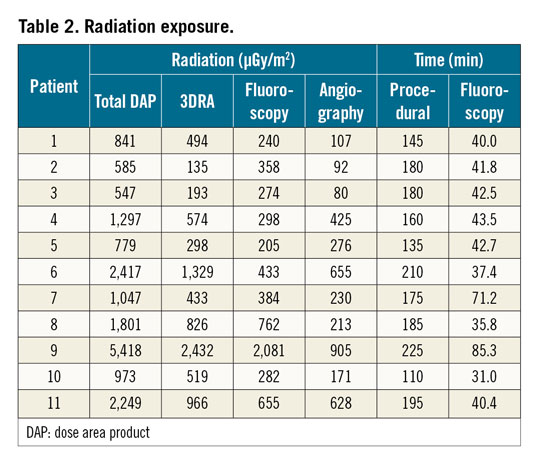
Median total dose area product (DAP) was 1,047 µGy/m2 (range 547-5,418), median procedural time 180 minutes (range 110-225) and median fluoroscopy time 41.8 minutes (range 31.0-85.3). In general, 3DRA DAP accounted for around half of the total DAP (Table 2).
Discussion
This paper is the first description of the oval stent technique. This novel technique successfully relieved LPA obstruction without increasing pre-existing or causing novel LMB compression in a series of 11 patients. Periprocedural 3D imaging with 3DRA visualises vessel-vessel and vessel-airway interactions in a single run, providing us with new insights on the effect of surgery and stenting on neighbouring structures, influencing clinical decision making in the paediatric catheterisation laboratory. Recent papers have confirmed the mass effect of stents in the mediastinum9 and limited retro-aortic space including the LPA and LMB after the Norwood or DKS procedure3,4,5. Thus, Fontan patients are at risk of airway compression during LPA stenting, especially those with a DKS7. Our results confirmed this observation as 11/51 (21.6%) patients who had undergone LPA stenting were at risk of or had pre-existing LMB compression, necessitating an oval stent procedure; six of them were palliated with a DKS anastomosis.
The oval stent technique was successful in relieving LPA obstruction in all patients, while maintaining airway patency in nine. Unfortunately, ovalisation had no immediate effect on airway patency in the two patients with pre-existing severe LMB compression. In patient 3 with known airway compression on CT, stent ovalisation as well as intrabronchial balloon dilatation during the same procedure showed no immediate effect on airway diameter. However, six months later bronchoscopy showed improved airway patency without recurrent pulmonary infections. Patient 11 suffered from LMB compression after stent placement of a subtotal LPA stenosis in a limited retro-aortic space. 3DRA of the airway showed no changes in airway diameter and balloon dilation of the bronchus was performed with manual ventral chest compression to modify dorsal stent shape. The end result with 3DRA showed a mild c-shaped stent (bent around the LMB) and an unchanged airway diameter (2.5 mm) despite reduction of the ventral-dorsal stent diameter from 10 to 9 mm creating 1 mm more space for the LMB. Hypothetically, time and patient growth may facilitate airway patency in the future as seen in children with LMB compression treated with posterior aortopexy12; however, this remains to be seen as follow-up for this patient was limited. It is questionable whether regaining airway patency after the age of 11, as in these patients, will be of clinical benefit since alveolar development is most pronounced at a young age13.
The oval stent procedure starts with optimal visualisation of the airway and cardiovascular anatomy, especially in the retro-aortic space. This can be done preprocedurally by CT or magnetic resonance imaging (MRI)14 or periprocedurally by 3DRA. Protocols for optimal 3DRA acquisition including airway visualisation have been published previously7,8. Our experience is that airway reconstruction may be difficult in case of surrounding large amounts of contrast. A non-contrast “empty” 3DRA run reproducibly resulted in high-quality airway reconstructions in our series and may easily resolve this problem. Empty 3DRA runs can be performed with a DAP as low as 10% of a conventional 3DRA. In addition, 3D reconstructions help to estimate target diameters of the oval stent, taking into account the surrounding structures.
When an LPA stent is already in situ, we prefer to ovalise this stent using a short venous sheath and a steerable long sheath. In this way both balloons and wires can be manipulated separately in an optimal manner. The steerable sheath allows both controlled superior-inferior positioning by tip deflection as well as anterior-posterior positioning by rotation. In case of two femoral venous access sites, we use the steerable sheath to position the inferior balloon as wires automatically tend to position in the roof of the LPA. The steerable sheath is available from as small as 6.5 Fr (Oscor Inc., Palm Harbor, FL, USA) and enables obtaining ideal deflection in the LPA ostium. With the balloon in the optimal position, inflation can be performed under biplane fluoroscopy with 3D reconstruction overlay. Balloons may be inflated simultaneously or consecutively. In case of a native LPA stenosis, balloon interrogation is mandatory and should be performed to confirm stable balloon position without airway compression. This can be performed with an empty 3DRA run. Thus, an old-fashioned bronchography with tracheal contrast injection can be avoided. Bronchoscopy has also been reported to guide LPA stenting but requires an additional specialist in the catheterisation laboratory10. In case of a patent airway during balloon interrogation, one can proceed with primary single balloon stenting followed by secondary ovalisation. In case of bronchial compression, we performed primary oval stent implantation using two smaller balloons. Primary oval stent implantation necessitates a larger single sheath and superior-inferior positioning of the stent may be difficult. In case of suboptimal superior-inferior positioning, only one of the balloons should be inflated, allowing secondary superior-inferior positioning of the balloons.
DAPs delivered to the patients in this series are comparable to DAPs reported in paediatric interventional cardiology15,16. This is in spite of the fact that multiple 3DRAs were performed in each patient. The effective radiation dose of one rotational angiography can be reduced dramatically to 0.54 mSv when a simple dose reduction protocol is applied17. Finally, 3DRA offers the opportunity to perform interventions with 3D reconstruction overlay. This facilitates wire, sheath and balloon positioning and reduces radiation.
3DRA has improved our understanding of the 3D relationship of heart, vessels and surrounding structures, making it indispensable in our catheterisation laboratory. Besides, it enables the technique of reshaping stents with multiple balloons, leading the way to more personalised or even more anatomically shaped interventions. Future research will determine whether this technique will be able to improve the long-term outcome of our patients.
Limitations
This study is limited by its single-centre design and small sample size. Future studies should investigate the oval stent technique in a larger patient group and its applicability in other congenital heart diseases.
Conclusions
The oval LPA stent technique with double balloon inflation was successfully used in 11 patients at risk of or with pre-existing bronchial compression after a DKS or Norwood procedure. Oval stent geometry guarantees LMB patency without increasing pre-existing compression. 3DRA is crucial for the understanding and interventional approach of vessel-airway interactions.
|
Impact on daily practice Left pulmonary artery (LPA) stenosis is common after cavopulmonary connection, but stenting may be complicated or contraindicated by left main bronchus (LMB) compression. Three-dimensional rotational angiography is indispensable. It enabled the development of our novel oval LPA stent technique that was successful in these patients guaranteeing airway patency without increasing LMB compression. This technique should be implemented into routine clinical practice as it results in more personalised and anatomically shaped interventions in the catheterisation laboratory. |
Conflict of interest statement
G. Krings is a member of the Siemens Advisory Board and a consultant for Edwards Lifesciences. The other authors have no conflicts of interest to declare.

