Abstract
Aims: Limited data exists on midterm results concerning paediatric interventions on stenotic or occluded systemic veins following indwelling lines, cardiac surgery, or catheterisations. The purpose of this study was to report our acute and intermediate results concerning patients with (Group A) and without (Group B) congenital heart diseases (CHD) over a 10-year period.
Methods and results: From January 2000 to December 2010, 32 patients (23 in Group A and nine in Group B, respectively) underwent 39 interventional catheterisations aimed to dilate or recanalise occluded iliofemoral veins, inferior or superior venae cavae. Initial and follow-up catheterisation data were reviewed retrospectively. Midterm results were evaluated by means of echography, angiography, and CT scan; in all 15 and 17 patients, respectively. Median age and weight of all patients at catheterisation were five years (range 0.1-18) and 15 kg (range 2-60), respectively. Fifty-two stents were implanted in 29 patients (32 vessels). In 25 patients 28 vessels were occluded and required recanalisation. There were no major complications. In all but three patients it was possible to treat the lesion. There were two procedural complications (5.1%): one acute stent occlusion and one local dissection. At a median follow-up of 2.5 years (range 1-10) we observed six complications of stenting (11.5%): two fractures, two occlusions and two restenoses.
Conclusions: Interventional catheterisation of stenotic or occluded systemic veins grants good immediate results at a low rate of complication. Stent dilatation or recanalisation may open the vessel for use during future procedures. However, long-term results are yet to be established.
Introduction
Stenotic lesions of systemic veins may occur in patients with and without congenital heart diseases (CHD). Repeated catheterisations, implantation of permanent central catheters or intracardiac pacemakers, and surgical venous anastomoses can lead to stenosis or occlusion of systemic veins1-3. Pressure elevation in the systemic venous district can prompt the development of anomalous venous connections, which are a possible source of cyanosis4,5. Interventional catheterisation using balloon dilatation and stenting is an effective technique for the treatment of a large number of anomalies. Reports on interventions on systemic veins in children concern a limited number of pathologies and a small series of patients. To the best of our knowledge, only one report examined the long-term results of interventions on superior vena cava stenosis2 and only one investigated the midterm results of recanalisation of occluded systemic veins in children6. The aim of this study was to review our experience concerning interventions on stenotic or occluded systemic veins in children with (Group A) and without (Group B) CHD over a 10-year period.
Population
From January 2000 to December 2010, 32 patients (23 in Group A and nine in Group B, respectively) underwent 39 interventional catheterisations in order to dilate stenotic veins or recanalise occluded vessels.
Inclusion criteria were: stenosis or occlusion of systemic veins precluding access to the heart for cardiac catheterisation, permanent central catheters or pacemaker implantation, stenotic lesions or occlusion of systemic veins accompanied by clinical manifestations.
Clinical characteristics of patients, including first diagnosis, surgical treatment, reasons for venous treatment and affected vessels are illustrated in Table 1.
Median age and weight of patients at catheterisation were five years (range 0.1-18) and 15 Kg (range 2-60), respectively. Among the 23 patients in Group A, 20 had undergone a complete repair or palliation, and three had been transplanted. Among nine patients in Group B, two exhibited cystic fibrosis, five manifested short bowel syndrome, one showed severe prematurity and one displayed chronic renal insufficiency. All needed either permanent central catheters for antibiotic treatment, dialysis or parenteral nutrition.
The study was approved by the institutional review committee. Parents of all children who had been informed that they would undergo echography specifically to allow the visualisation of systemic veins, a further catheterisation or a CT scan, gave their written consent.
Methods
Hospital records and catheterisation files were reviewed to identify associated malformations and complications occurring during interventional catheterisation.
Midterm results were evaluated with echography for all patients, using angiography for those for whom a second intervention was planned (15 patients) and by CT scan for those for whom a non-invasive morphological evaluation of the heart was necessary (17 patients).
All children had clinical evaluation, cardiac catheterisation, multislice CT scan and echographic controls at the Paediatric Hospital Regina Margherita, Turin, Italy.
Cardiac catheterisation
The technique of catheterisation did not differ from that previously described1-6, however the variety of lesions and the unique vascular anatomy of patients with multiple vascular occlusions often required several and atypical vascular accesses, including transhepatic and collateral vessel access. Heparin at the dose of 50 UI/Kg and antibiotic prophylaxis were administered. Tight stenotic lesions were predilated using Tyshak™ or Ghost™ (NuMED Inc, Hopkinton, NY, USA) or BALT (Balt, Montmorency, France) balloons. To cross occluded vessels we used coronary guidewires with a soft tip and a good support, such as Whisper Extra Support (Abbott Vascular, Santa Clara, CA, USA) or guidewires for chronic total occlusion, such as Miracle or Confianza (Abbott Laboratories, Abbott Park, IL, USA). We generally used balloon dilatable stents (Genesis™ stent; Cordis/Johnson and Johnson, Warren, NJ, USA, and CP™ stent; NuMED Inc). Premounted Genesis™ stents, dilatable up to 12 mm were favoured for iliofemoral vessels. Genesis XD™ stents (dilatable up to 18 mm) and CP™ stents (dilatable up to 25 mm) were used for the superior vena cava (SVC) and the inferior vena cava (IVC), respectively. We employed auto-expansible stents (S.M.A.R.T.™ stent; Cordis/Johnson and Johnson) in two patients in whom a new central catheter was implanted in a collateral vessel. Post-dilatation was performed when incomplete stent dilatation had been obtained by using high-pressure balloons (Powerflex™; Cordis/Johnson and Johnson or Mullins™; NuMED Inc.). After stent implantation patients received heparin during 24 hours and aspirin during six months. Patients with a single ventricle were maintained under aspirin or anticoagulant treatment.
Echography
The patency of the treated vein(s) was evaluated by ultrasound using a Convex 5 MHz probe and a Philips IE33 echocardiography system (Philips Medical Systems, Eindhoven, The Netherlands). We assessed all patients according to major and minor ultrasound criteria for deep venous thrombosis7 medical history, and clinical data.
Multislice CT scan
All examinations were performed with a 64-slice CT scan (LightSpeed™ VCT; GE Medical Systems, Milwaukee, WI, USA). The standard acquisition protocol included 400 ms rotation time, 64×0.625 mm slice thickness, pitch 0.984, voltage 80 kV, current varied during acquisition and adapted to the weight of the child. A bolus of 1.5 ml/kg injection of iodine contrast medium (lohexol 300 mgI/ml; Schering SA, Berlin, Germany) was injected at a rate of 0.5 to 1.5 ml/s with a power injector (EnVision CT™; Medrad, Pittsburgh, PA, USA), followed by a chaser bolus of 5 to 10 ml of saline. Patients over six years of age were instructed to inspire and hold their breath during data acquisition. Younger patients were allowed to breathe freely.
We performed acquisition at 75% R-R interval, and completed reconstructions at every 10% R-R interval. Data sets were transferred to a dedicated workstation for post processing and evaluation and analysed by independent investigators with experience in cardiac and congenital CT, blind to the result of the catheterisation. Post processing tools, such as multi-planar reconstruction and maximum-intensity thin slab projection were used in all cases in order to define the anatomy and obtain projections corresponding to standard angiographic views.
Statistical analysis
Descriptive statistics for the total population were obtained. Age and weight were quoted as a median and a range; continuous variables were presented as the mean±standard deviation. Differences between groups were tested for categorical variables using the Fisher’s exact test. Differences in continuous variables were tested using the Wilcoxon rank sum test. Linear regression analysis was performed using the correlation coefficient “r”. All statistical analyses were performed using the R software package (http://www.R-project.org; The R Foundation for Statistical Computing, c/o Institute for Statistics and Mathematics, Vienna, Austria). Statistical significance was considered as p-values less than 0.05. All tests were two-sided.
Results
IMMEDIATE RESULTS
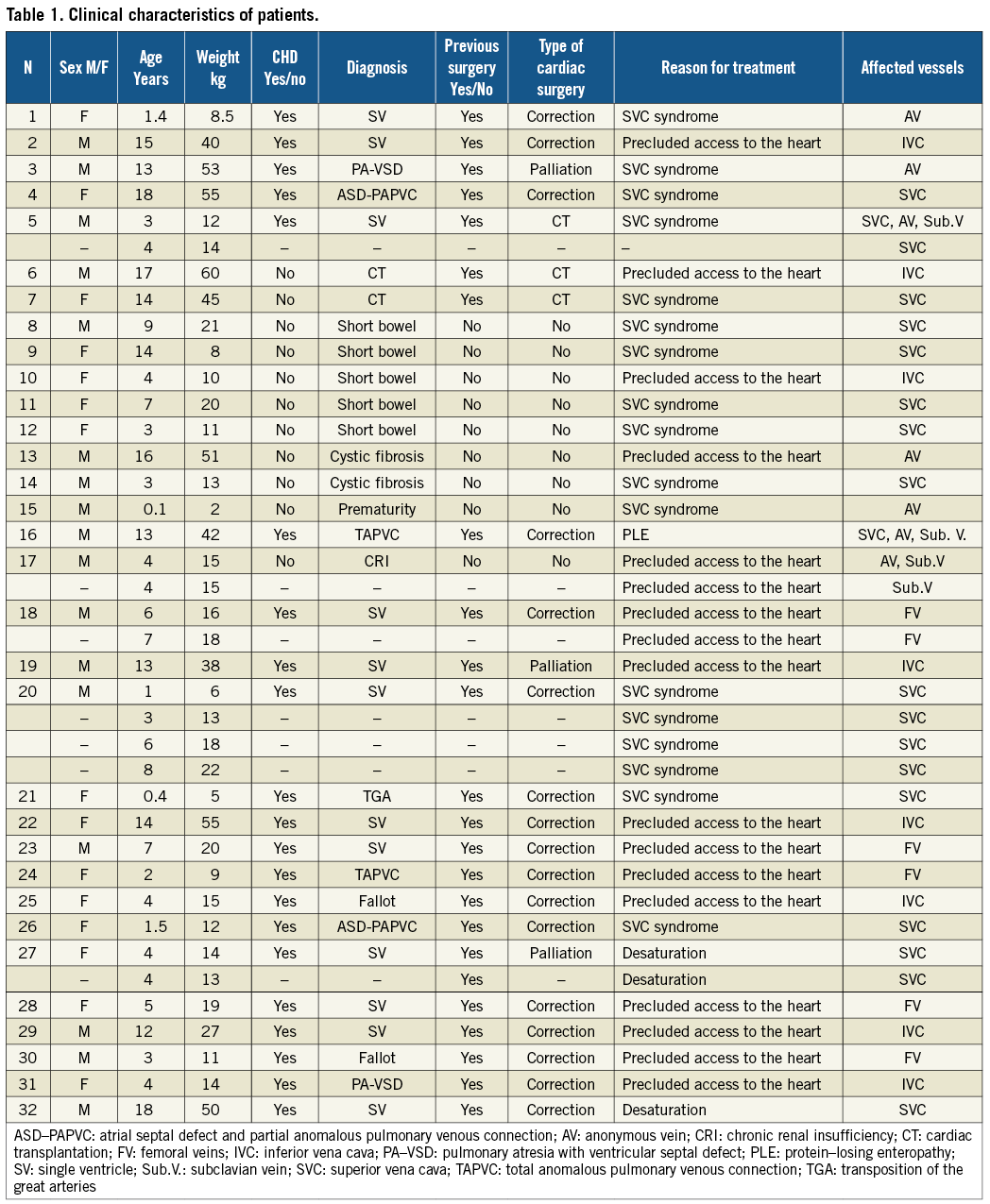
Catheterisation data and outcome are shown in Table 2. Fifty-two stents were implanted in 29 patients (32 vessels). In 25 patients 28 vessels were occluded and required recanalisation (Figure 1).
Figure 1. A child with a repaired total anomalous pulmonary venous connection and bilateral stenosis of pulmonary veins (patient 24) had complete occlusion of the iliofemoral veins, precluding access to the heart (A). The right iliofemoral axe was recanalised using two premounted Genesis stents (B).
In our series there were 23 patients in Group A and nine in Group B, respectively. Three out of 23 patients in Group A had been transplanted. Group A patients presented stenoses or occlusions of systemic veins in the superior and inferior part of the body; Group B patients exhibited stenoses or diffused occlusions of systemic veins in the superior part of the body.
Predilatation was performed in 18 and three patients in Groups A and B, respectively; post-dilatation was necessary in 15 and nine patients, respectively.
Dilatation by stent was generally effective. Mean gradient through the stenotic area diminished from 8.1±2.8 to 0.2±0.6 mmHg, p<0.001. The reduction of the gradient was similar in Group A and Group B patients. There were three failures (5.8%): two in Group A and one in Group B. Two of the failures were due to complete occlusion of the SVC, jugular and subclavian veins (patients 11 and 16) and one due to complete IVC occlusion, including the intra-hepatic segment (patient 31). In this patient the inferior venous drainage attained the azygos vein above the diaphragm. There were two procedural complications (3.8% of the overall population), both in Group A patients: one immediate stent occlusion (patient 18) and one vessel dissection (patient 25). Group A patients underwent eight associated procedures: one atrial septal defect closure, one atrial septostomy, one Fontan’s fenestration closure, four implantations of a new pacemaker and one stenting of pulmonary arteries. In Group B patients, four implantations of permanent central catheters were performed. There were no procedural deaths.
During intermediate follow-up, four patients in Group A and one in Group B needed a further catheterisation and required additional interventions on the affected vessel (Table 2).
In our series, seven and six children of Group A and B, respectively, developed a SVC syndrome associated with headache, dyspnoea, and swelling. One patient (number 16), who had had a correction of total anomalous pulmonary venous connection and a pacemaker implantation, showed complete occlusion of the SVC, jugular and subclavian veins. Ten years after operation, he developed protein-losing enteropathy and needed diuretics and recurrent protein infusions. Among transplanted patients, one with IVC stenosis exhibited hepatic congestion that completely regressed after percutaneous treatment.
One patient having had a Fontan-type repair died eight months after catheterisation from the dysfunction of the single ventricle, and one premature patient died one month after catheterisation, from pulmonary infection.
Midterm results
Two overseas patients were lost at follow-up. At a median follow-up of 2.5 years (range 1-10) we observed six silent complications of stenting (11.5% of the overall population). All occurred in Group A patients: two fractures (one with rotation in the venous lumen), two occlusions and two restenosis. Stent stenosis, occlusion or fracture were observed only in vessels measuring <6 mm, such as anonymous or jugular vein and in a tiny SVC (patient 27 with wide left SVC) (Figure 2). The remaining stents evaluated by echography (Figure 3), angiography or CT scan (Figure 4 and Figure 5) had a regular profile and were patent.
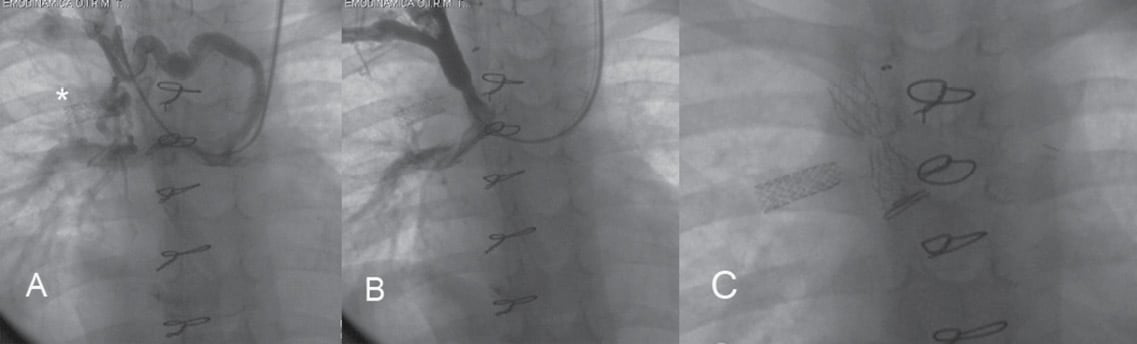
Figure 2. A child with a bilateral cavopulmonary connection (patient 27) and a previous stent implantation in the right superior pulmonary vein (*) had complete occlusion of the right superior vena cava and an abundant collateral circulation accompanied by profound cyanosis (60%) (A). Superior vena cava was recanalised using a Genesis stent, which could not be completely dilated (B). At a further angiographic control a transversal stent fracture was evident (C).
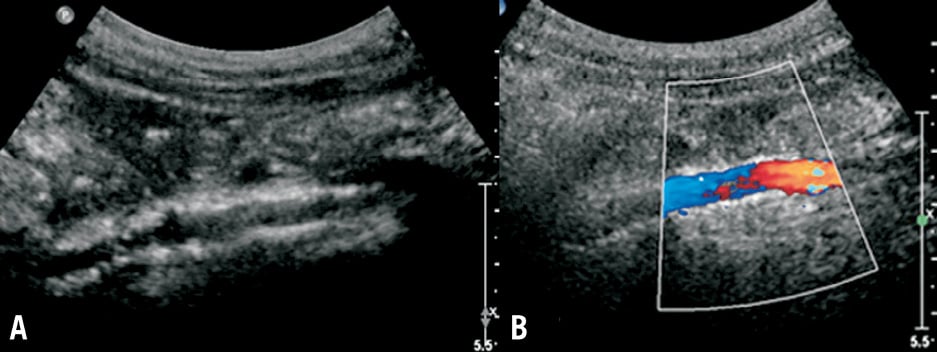
Figure 3. The bidimensional echographic control of a recanalised inferior vena cava (patient 19) demonstrated the stent’s patency (A). The stented inferior vena cava is small, but has a laminar, non-turbulent flow in colour Doppler four years after recanalisation (B).
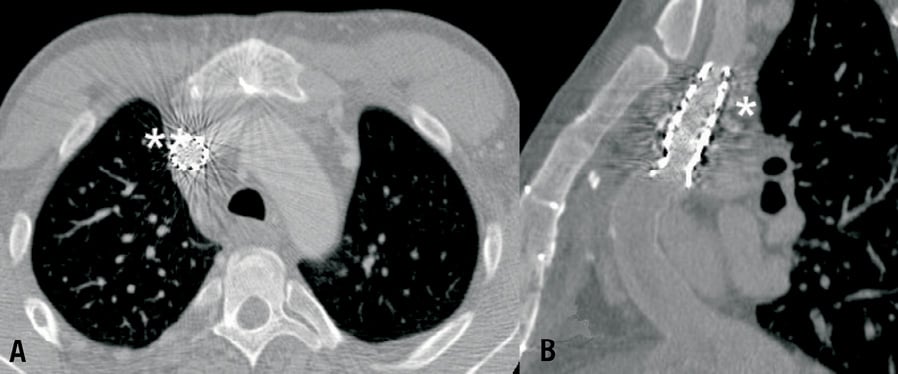
Figure 4. Patient 32. CT scan evaluation of recanalised superior vena cava in short axial (A) and long axial view (B).
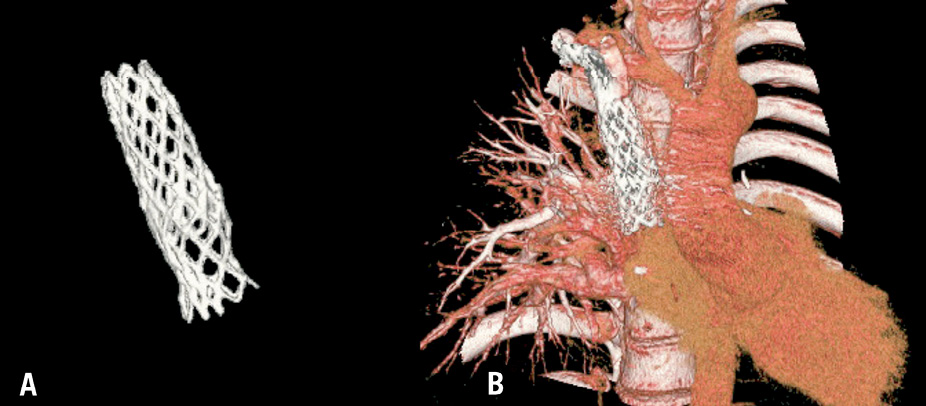
Figure 5. Same patient as in Figure 4. Three-dimensional stent reconstruction with (A) and without subtraction of adjacent structures (B).
We could not find any correlation between age, weight of patients, presence of CHD, type of procedure performed, type of vessel treated, number and type of device implanted and occurrence of early and/or late complications. However, the sample was relatively small.
Technical section
To treat stenoses of systemic veins, we evaluated the diameter of the stenotic vessel. If the diameter of the stenotic site was <4 mm, or in the presence of a calcific lesion, we performed a predilatation in order to avoid stent migration during deployment. If, after stent implantation, a waist at the stenotic site persisted, we performed a post-dilatation with high pressure balloons.
For total chronic occlusion of the SVC, we attempted to cross the occluded segment in an antegrade or retrograde fashion, in accordance with the anatomy. We generally used guidewires for chronic total occlusion. We then created a jugular to femoral vein circuit using a snare. If the occluded segment was longer than 5 mm we performed a predilatation. A stent was then implanted and a post-dilatation was performed if a waist persisted.
For total chronic occlusion of iliofemoral veins and IVC we attempted to cross the occluded segment via a femoral venous approach, using coronary guidewires with a soft tip and a good support, or guidewires for chronic total occlusion. If the obstructed native vessel was crossed, a predilatation was performed. If it was impossible to cross the occluded segment of the native vessel, the guidewire was advanced through the collateral circulation. One or more stents were then implanted either in the native vessel, or far enough in the collateral vessel to reach the native, and a post-dilatation was performed when an incomplete stent expansion was obtained.
Discussion
The fate of stents implanted in vessels with non-pulsatile, low-pressure flow has not been established in large series of patients. This study demonstrates that interventional catheterisation of stenotic or occluded systemic veins grants good immediate results at a low complications rate. Stent dilatation may ameliorate symptoms and open the vessel for use during future procedures. Although previously placed stents can be safely recanalised if necessary, stent thrombosis raises concern in midterm results. We show that stent occlusion is a relatively frequent event, mostly when stents are implanted in small vessels, or when there is a competitive flow. We observed stent occlusion only in vessels measuring <6 mm. Other authors reported that a small vessel can favour stent occlusion6.
CHD patients (Group A)
Stenosis or occlusion of the iliofemoral veins and/or the inferior and superior venae cavae can occur in patients with CHD after perioperative indwelling lines, cardiac catheterisations and extracorporeal membrane oxygenation (ECMO) cannulation. Long-term perioperative indwelling lines and large sheaths during cardiac catheterisation are the most likely aetiologies for stenoses and occlusions8-10. Stenoses and occlusions rarely cause symptoms in children. Symptoms may be attenuated by the development of venous collaterals; however, venous collateralisation may not be completely benign, as formation of paravertebral collaterals has been shown to cause nerve compression11.
In addition, CHD children often require systemic venous vessels for access during cardiac catheterisations or subsequent surgeries. As more children with CHD survive until adulthood, symptoms from venous stenoses or occlusions may become apparent11. CHD patients with a pacemaker can develop occlusions of the subclavian vein7. These patients may need recanalisation of the occluded vessel for further pacemaker implantation12,13.
Few reports exist concerning the fate of suture lines on superior and inferior venae cavae in transplanted patients. Venous anastomotic stenoses have occasionally been described2,3. In children, the need for repeated biopsies leads to the progressive loss of venous access. In a paediatric population, cardiac biopsies should be performed via a long venous sheath from the groin and non-invasive methods to assess the presence of rejection should be encouraged.
Non-cardiac chronic diseases (Group B)
Patients with chronic diseases in need of nutrition or drug infusion through central catheters progressively lose peripheral and central vascular accesses and develop an abundant, superficial and fragile collateral circulation, which is often unable to provide free venous blood return to the right atrium. Reopening central vessels of the superior part of the body is particularly important in these patients, either to treat a possible superior vena cava syndrome or to make the insertion of new central catheters possible before lung, liver or bowel transplantation14,15. The results of interventional catheterisation in this subgroup of patients can be disappointing, due to the extension and longevity of vascular thrombosis. The necessity of adequate material to attempt perforation of occluded vessels and maintain their patency is mandatory1,13. In our experience, stenting of wide collateral vessels can provide a good venous discharge and allow the insertion of central lines in selected patients14. Other authors reported the need of radiofrequency perforation to recanalise an occluded SVC13.
In accordance with our results, previous reports indicated that stent implantation is a safe technique and that patency is longstanding in the vast majority of patients. In-stent intima proliferation is rarely noted in patent vessels at midterm follow-up6. However, after a longer-term follow-up complete occlusion may be expected. We evaluated all patients by means of echographies, which proved to be an easy, non-invasive, not irradiating and rapid method for the analysis of stent patency (Figure 3).
Patients who needed a morphological study of the heart underwent CT scan, which also proved to be a useful method to evaluate the vein patency and stent restenosis or occlusion (Figure 4 and Figure 5).
The study is limited by the length of follow-up. Long-term follow-up will be necessary to ensure that vessels can continue to be re-dilated to adult size. The long-term outcome of patients with systemic venous stents compared with patients with unstented stenoses and occlusions will help determine whether or not stent placement is indicated for a larger number of patients.
Conclusion
Our results suggest that the patency of systemic veins should be regularly checked in children having undergone several cardiac catheterisations. In our centre we perform a vascular echography only once per year, in order to limit the radiation dose. We use heparin during any venous catheterisation at the dose of 50
or 100 UI/kg to preserve the vessel’s patency, and we try to employ small sheaths and limit the number and duration of catheterisations.
Stenoses or occlusion of systemic veins can successfully be recannulated and stented without complications. Intermediate-term results indicate that the majority of vessels remain patent and can be used for subsequent catheterisations or indwelling lines. In our experience balloon-expandable stents should be preferred to treat tight venous stenoses. In-stent restenosis or occlusion can occur, but responds to further balloon angioplasty. Insufficient distal inflow may be a risk factor for stent occlusion. The use of longer-term aspirin or chronic anticoagulation may prevent the recurrence of venous occlusion; however in a paediatric population the risk-benefit ratio should be considered. Non-invasive methods to assess cardiac and vascular anatomy in children should be encouraged.
Acknowledgements
We thank Gregorio Curello for the English revision of the manuscript.
Conflict of interest statement
The authors have no conflict of interest to declare.

