Abstract
Aims: The VALEO® vascular stent is a stainless steel, pre-mounted, open-cell stent. Redilation to large diameters and low profile are advantages in growing children. Clinical experience is scarce. This study aimed to analyse our experience with the use of the VALEO® vascular stent in children.
Methods and results: Between June 2010 and December 2012, 41 VALEO® stents were inserted during 30 transcatheter (group 1) and three perioperative procedures (group 2). Data were retrospectively analysed. Median age at implantation was 3.8 years (four days - 23 years), and median weight was 13.3 kg (2.2-53 kg). Indications were: pulmonary artery (n=23), pulmonary vein (n=1) or subhepatic vein (n=1) stenosis, and ductus arteriosus stenting (n=8). Stent placement was achieved in all but one. Acute complications (n=11) included stent dislodgement in two patients and fracture in three patients, with vessel predilatation as a risk factor. Median “stent” follow-up reached 7.83 months (0.4-34.3 months) and included 26 recatheterisations (23 patients, median interval 6.2 months, range 0.2-33 months). Ten stents were redilated. Surgery in six patients (interval 1.9 to 10 months) showed patent endothelialised stents. No “late” type II or III stent fractures were seen.
Conclusions: The VALEO® stent is useful in children. Low radial force is counterbalanced by high flexibility, allowing implantation in distal and tortuous lesions. Early fractures occur. Longer-term follow-up is needed.
Introduction
In recent years, stent placement has assumed an important and still increasing role in the management of children with congenital heart disease. Variety in cell design and material have improved the characteristics of the currently available stents remarkably and made their use in children much easier and safer. Stents are currently available in various lengths and maximal diameters. Their flexibility has improved and the pre-mounted stents have better profiles. Several stents are redilatable, which allows them to adapt better to the growth of the children1,2.
The VALEO® stent (Bard Inc., New Providence, NJ, USA) was initially designed for biliary use (VALEO® biliary stent). The use of the stent has been extended to atherosclerotic vascular lesions (VALEO® vascular stent). It is an open-cell, stainless steel stent, pre-mounted on a low-profile, high-pressure balloon. The balloon diameters range between 6 and 10 mm (1 mm increments) and the stent length between 17 mm (18 mm for the stents mounted on the smallest balloons) and 56 mm (17-18, 28, 36 or 56 mm).
The main advantages are the low profile, the flexibility and the possibility to dilate the stent to large diameters without shortening3. Experience in patients with congenital heart disease, in whom the use of this stent is still “off-label”, is scarce3-5. We sought to analyse retrospectively our experience with the VALEO® vascular stent in growing children with cardiovascular lesions.
Materials and methods
PATIENT POPULATION
Between June 2010 and December 2012, 41 VALEO® stents were implanted during 33 procedures in 32 different patients. The procedure was mostly transcatheter (30 procedures in 29 patients, group 1) but in three patients the stent was inserted perioperatively (group 2). Median age at the time of stent implantation was 3.8 years (range 4 days to 23 years), and the median weight was 13.29 kg (range 2.2 to 53 kg).
Initial cardiac diagnoses, indications for stent placement and stent locations are summarised in Table 1.
Pulmonary artery hypoplasia or stenosis (n=23) was the major indication. This population included the three patients (two with tetralogy of Fallot and one with pulmonary atresia and ventricular septal defect) in whom the stent was inserted perioperatively (group 2).
The second major indication was stenting of the arterial duct as part of a hybrid approach for the initial palliation of patients with hypoplastic left heart syndrome (HLHS) or “borderline” HLHS, or patients with interrupted aortic arch. In our institution, we currently favour the hybrid approach (surgical banding of the pulmonary arteries and ductal stenting) as initial palliation in patients with confirmed or borderline HLHS. Neonates with interrupted aortic arch usually undergo primary complete repair but, in the presence of risk factors, we also favour a hybrid palliation. The two patients in our study with interrupted aortic arch had microdeletion in 22q11. The option of primary palliation was taken because of the small weight (2.2 kg) for one patient and because of enterocolitis for the other one. For logistic reasons the patients first underwent the surgical banding of the pulmonary arteries in the operating room and, subsequently, within the following days, ductal stenting was performed in the catheterisation laboratory, using femoral venous and arterial access.
The patient with pulmonary vein stenosis was a pre-term baby with severe bronchopulmonary dysplasia. Cardiac surgery to relieve the bilateral pulmonary vein stenosis was contraindicated from a pulmonary point of view. The patient underwent multiple pulmonary vein stenting while on the waiting list for heart-lung transplantation.
TRANSCATHETER STENT INSERTION (GROUP 1, N=30)
Informed consent of the parents and/or patients was always obtained. Catheterisation was performed under general anaesthesia in all patients. Venous and arterial access was obtained, according to the planned procedure. The venous access was usually femoral. In three patients with cavopulmonary shunt, jugular venous access was mandatory in order to reach the pulmonary arteries. In one patient, the jugular venous access was chosen as femoral venous access failed. Transhepatic venous access was elected in the patient with hepatic vein stenosis. All patients received heparin (100 U/kg) once venous and arterial access had been obtained. Heparin (50 U/kg) was repeated after two hours if needed. Haemodynamic and angiographic assessments, as needed for the procedure, were first obtained.
In case of vessel stenosis (pulmonary artery, pulmonary vein, subhepatic vein) the vessel was predilated if needed, according to the operator’s clinical judgement. Predilation was performed with medium-, high- or ultra-high-pressure balloons. The stent length and the balloon size were chosen according to the usual rules, in order to normalise as much as possible the vessel diameter, avoiding overdilation of the vessel. The length of the stent was chosen according to the total length of the stenosis or hypoplasia, even if this implied covering of side branches. A long sheath was advanced up to the stenosed vessel, in order to protect the balloon-stent ensemble. The smallest possible long sheath diameter was always chosen (6 or 7 Fr), according to the manufacturer’s recommendations. In a few very small patients with very high right ventricular pressures, it was deliberately decided not to use a long sheath in order to minimise haemodynamic compromise. The stent was in those cases advanced “unguarded” over the wire. Post-dilation of the stent was performed if there was residual stenosis or if the wall apposition of the stent was considered insufficient.
In case of ductal stenting (hybrid approach) the prostaglandin infusion was usually stopped four to eight hours before the expected start of the procedure. Aortography was first performed to analyse the morphology of the duct and the size of the descending aorta. The stent length was in these cases always 17/18 mm for short ducts and 26 mm for longer ducts. The diameter of the balloon was chosen to fit the diameter of the descending aorta. A wire was first inserted in an anterograde way through the duct. The wire was in some cases snared at its distal aortic end to increase stability. The pre-mounted stents were advanced over the wire without the use of a long sheath in order to minimise the haemodynamic interference in these very unstable children. Particular care was taken to advance the balloon-stent ensemble at the level of the tricuspid valve to avoid dislodgement at this level.
SURGICAL STENT INSERTION (GROUP 2)
Informed consent of the parents and/or patients was always obtained. The stent placement was performed under cardiopulmonary bypass, as part of the total repair for two patients with tetralogy of Fallot and at the time of conduit change in one patient with Patr VSD. Stent selection (balloon diameter and length of the stent) was based on preoperative CT-scan or angiographic assessment. The stents were directly implanted without fluoroscopy guidance. Also, they were always secured by the surgeon with a single proximal stitch and flared at their proximal end.
DATA ANALYSIS AND STATISTICS
Patients’ clinical data, procedural characteristics, complications and follow-up data were retrospectively analysed. Follow-up was based on the clinical judgement of the attending paediatric cardiologists. Full clinical assessment, ECG and echocardiography were regularly performed in most patients. CT scan and repeat cardiac catheterisation were performed when indicated, based on clinical and echocardiographic evolution and according to the initial treatment strategy.
For the patients in whom the stent was inserted with the aim of remaining “permanently” (e.g., pulmonary arteries), the date of the latest clinical assessment was considered as the latest follow-up. For the patients who had ductal stenting (hybrid approach), the date of surgical treatment with arch reconstruction or the date of death, if before surgery, was used (“stent” follow-up). The date of the heart-lung transplant was used for the patient with multiple pulmonary vein stenosis.
Univariate analyses were performed using the chi-squared test (or Fisher’s exact test if necessary) to test relationships between qualitative variables. Multivariate analyses were performed using logistic regression models to assess factors associated with acute complications. All analyses were performed using R3.1 software (Copyright (C) 2013 The R Foundation for Statistical Computing, Vienna, Austria).
Results
ACUTE PROCEDURAL RESULTS
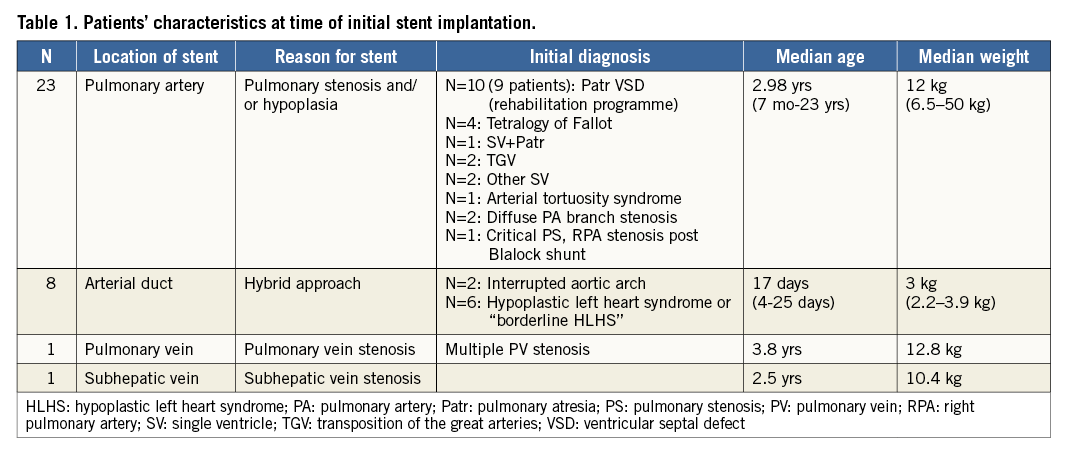
Predilation of the lesion was performed in 14 out of the 33 procedures (42%). One predilation was on a pulmonary vein stenosis and the remaining 13 on pulmonary arteries. The selected stents had balloon diameters ranging between 6 and 10 mm. The initial stent length was 17/18 mm (n=23), 26 mm (n=17) or 36 mm (n=1). For the eight patients in whom stents were inserted in arterial ducts (hybrid procedures), the length was 17/18 mm in four patients and 26 mm in the four other patients. The VALEO® stents could be implanted in all except one patient. This patient had arterial tortuosity syndrome characterised by severe hypoplasia, stenosis and tortuosity of the pulmonary arteries that made it impossible to advance and deploy the stent properly, despite the use of a guiding catheter. The guiding catheter could not cross the lesion and, while trying to advance the pre-mounted stent through the very narrow and tortuous vessel, the stent slid from the balloon and could not be placed correctly in the pulmonary artery. This stent could however be secured and finally deployed in the inferior vena cava (Figure 1). In seven patients, two VALEO® stents were implanted, most often in different locations. In six patients, other additional stents were inserted, either in side branches or in other locations. This includes a covered stent inserted in one patient after dissection was noticed after the VALEO® stent deployment. The open-cell design of the VALEO® stent allowed easy implantation of other stents across the cells (Figure 2). Vessel expansion was considered sufficient in most cases and post-dilation was only performed in five cases (all PAs).
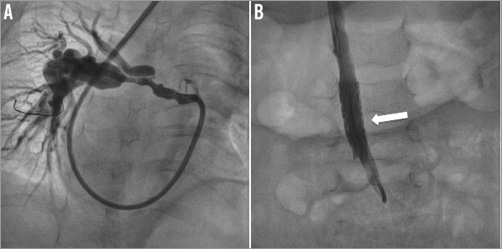
Figure 1. Angiographies in a patient with arterial tortuosity syndrome in whom we planned to implant a VALEO® stent in the right pulmonary artery. The left panel (A) shows the very stenosed and tortuous right pulmonary artery in which the stent could not be advanced. The stent was finally deployed in the low IVC (B).
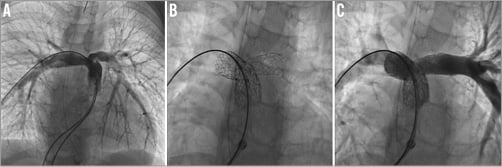
Figure 2. Proximal left and right pulmonary stenosis. A) A VALEO® stent is first inserted across the proximal LPA stenosis. The stent protrudes in the MPA and jails the RPA. The second stent (maxi-LD stent) is delivered into the proximal RPA, across a predilated cell of the VALEO® stent (B). Final result is shown on the right panel (C).
COMPLICATIONS
Acute complications occurred in 33% (11/33) of the procedures and were as follows: one reperfusion syndrome, three cases of haemoptysis, two cases of transient low cardiac output (including one patient who had concomitant open heart surgery), three stent fractures and two stent dislodgements. All the complications were seen in the PA group.
One stent fracture occurred in a very stenosed RPA in a patient with Patr VSD who previously had a right modified Blalock-Taussig shunt inserted. The stenosis was resistant and predilated with an ultra-high-pressure balloon. The stent fracture was noticed immediately following stent implantation. Small wall dissection and aneurysm formation were noticed and required covered stent implantation with subsequent satisfactory angiographic appearance. The other fracture occurred in a patient born with DORV and pulmonary atresia presenting conduit and proximal RPA stenosis. The VALEO® stent mounted on an 8 mm balloon was inserted in the proximal RPA without difficulties. Despite a good result, the stent was noticed to be fractured, without dissection or significant obstruction. The third stent fracture occurred in a surgical patient (group 2) at the time of postoperative stent dilation. The stent fracture was not associated with obstruction or vessel damage and was left untreated.
One stent dislodgement, which occurred in a patient with arterial tortuosity, has been described earlier. The other dislodgement occurred in a patient with Williams-Beuren syndrome with severe hypoplastic pulmonary arteries and suprasystemic right ventricular pressures. The choice was made not to advance a long sheath to reduce the haemodynamic compromise. However, the stent slipped from the balloon at the level of the tricuspid valve. The stent could not be retrieved. It was first decided to expand the stent slightly at the tricuspid level, towards the apex of the RV, which then allowed the advancement of a long sheath and deployment of other stents in the distal pulmonary arteries with subsequent significant reduction in the right ventricular pressures. The stent captured in the tricuspid valve was surgically removed, without any need for valve repair.
Reperfusion syndrome, haemoptysis and low cardiac output (6/33, 18%) were thought to be “disease-related” and indicated the severity of the pulmonary artery abnormalities. The stent fractures and stent dislodgements (5/33, 15%) were considered “stent-related” complications. Complications were more frequently seen in patients who required predilation of the pulmonary arteries. The relationship between predilation and complication was significant in the pulmonary artery group in univariate analysis (p<0.05). Multivariate analysis failed to show any significant risk factor.
FOLLOW-UP DATA
All patients were treated with IV heparin or low molecular weight heparin at least during the first 24 hours following stent insertion. This was changed for aspirin (5 mg/kg) as soon as possible. Aspirin was maintained for at least six months or until stent removal (hybrid procedures).
Four patients died, all with hypoplastic left heart syndrome (hybrid approach), before the second stage surgery, as a result of right ventricular failure. “Stent follow-up”, as defined earlier, reached a median of 7.83 months (range 0.4 to 34.3 months) for the whole cohort. For the PA group alone, follow-up reached a median of 13.4 months (range 3.7 to 34.3 months).
During follow-up, a total of 26 catheterisations were performed in 23 patients (72%). The first recatheterisations (n=23) were performed a median of 6.2 months (range 0.2-33 months) after VALEO® stent implantation. This includes the three patients from group 2, for whom it was decided to perform a cardiac catheterisation electively within the first months following surgery. The indications for recatheterisation in the other patients were either preoperative assessment, or based on echocardiographic data (increase of RV pressures) and/or lung-perfusion scan data or finally as part of the rehabilitation strategy in pulmonary atresia patients. One patient in the surgical group had one additional catheterisation 11 months after the first one. One other patient had two other cardiac catheterisations as part of a rehabilitation programme, 8.5 months and 13 months, respectively, after the initial VALEO® stent implantation.
In the three patients from group 2, further balloon dilation of the stent was performed to improve wall apposition. This resulted in acute fracture of the stent in one patient. This patient was recatheterised 11 months later and the stent redilated without any acute problem, in order to catch up with growth.
In one patient with borderline hypoplastic left heart syndrome, the VALEO® stent had completely closed off due to intense intimal proliferation. This could be explained by progressive evolution towards a biventricular circulation and “functional” exclusion of the arterial duct. Nevertheless, the child had persistent post-capillary pulmonary hypertension and finally underwent a successful Norwood-Sano intervention. Four other patients presented significant intimal proliferation of the stent and needed redilatation (Figure 3). Two other patients had redilatation of their stent to catch up with growth. In all the other patients, the VALEO® stent was nicely patent and did not need reintervention. The redilations of the VALEO stents were all performed with medium- or high-pressure balloons and with diameters at the most 2 mm larger than the initial balloon diameter. None of the stents has so far been dilated up to a large diameter (>15 mm).
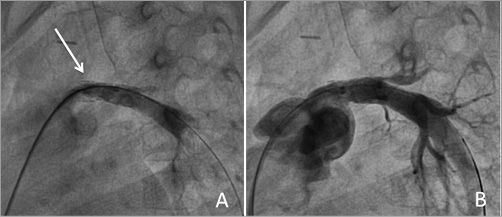
Figure 3. Lateral angiographies in a stented left pulmonary artery. Panel A shows mild endothelial proliferation at the proximal end of the stent (white arrow). The proliferation might have been favoured by mild residual stenosis at mid-stent level. Panel B shows good response after balloon dilation.
Six patients had surgery performed which allowed visualisation of the stent. In five, the stent was correctly endothelialised and patent (Figure 4). The remaining patient was the one described above with complete occlusion of the stent due to functional exclusion of the ductus.
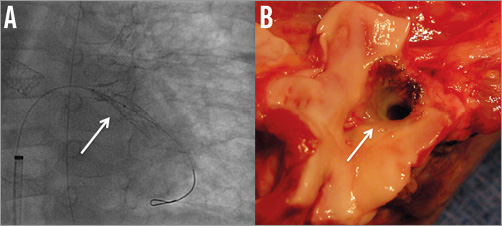
Figure 4. Fluoroscopic and macroscopic aspects of stents inserted in a left pulmonary vein. The left panel shows the VALEO stent (white arrow) inserted in the left lower pulmonary vein (A). The right panel (B) shows the explanted lungs with the VALEO stent in the vein (white arrow) nicely patent.
During follow-up, 27 patients (84%) had chest x-ray, fluoroscopy (cardiac catheterisation) or CT scan performed. None of the patients presented “late” type II or type III fractures. Type I fractures were very difficult to recognise or to rule out, due to the design of the stent cells (Figure 5).
Figure 5. Chest x-ray showing how difficult it is to ascertain absent or present fracture on a simple chest x-ray.
Discussion
The ideal stent for use in growing children is still unavailable. To reach perfection, stent characteristics should associate low profile, high flexibility, durability, radial force and strength and have the possibility to be dilated to large diameters. The spectrum of lesions to stent in patients with congenital heart defects is however very large. The ideal stent for treatment of coarctation in a teenager will be different from the ideal stent for tortuous arterial duct stenting in a newborn. The paediatric and congenital interventional cardiologist will need a “range” of stents on the shelf to face different clinical situations1.
The VALEO® stent is a balloon-expandable stent mounted on a low-profile flexible angioplasty balloon, initially designed for biliary use. The stent is laser cut into a 316 L stainless steel tube, using an open lattice design. The open-cell design is responsible for very limited foreshortening if the stent is dilated progressively3. The major advantages of the pre-mounted VALEO® stent in children are the low profile, the flexibility, the open-cell design that facilitates further access and treatment of side branches, and the potential for further dilation up to large diameters without shortening. These advantages are all illustrated in our study. The low profile and high flexibility were very advantageous in our small patients and in the small vessels. The open-cell design allowed access and treatment of other side branches or of the contralateral pulmonary artery in some patients. Redilatation of the stent has been successfully performed in a few patients but our study follow-up is too short to analyse long-term redilatation possibilities in growing children properly. In addition, none of the stents has so far been dilated to a large diameter (>15 mm). We used the stent in various locations, and this versatility of the stent may be an advantage for paediatric units which have a limited access to the stents that are currently available on the market.
Stents with open-cell design are considered to have lower radial force than stents with closed-cell design. In our population, characterised by young age and the variety of diseases, the stent was able to open up the lesions in an adequate way with sustainable results at follow-up. It is, however, important to note that most of the patients with tight pulmonary artery lesions had the lesions “prepared” before stent insertion using medium- or high-pressure balloons, as part of intense pulmonary artery rehabilitation programmes in patients with very abnormal pulmonary arteries6,7.
Two stent dislodgements were encountered in our study, both in patients with difficult pulmonary artery lesions. This suggests that the stent is not reliably mounted on the balloon. Although we have, in our unit, advanced the stents “unprotected” (without the use of a long sheath) in some circumstances such as ductal stenting in neonates (hybrid approach) and in case of very high right ventricular pressures, this is something that we do not recommend any longer. The stent needs to be advanced inside a long sheath to secure the balloon-stent ensemble and to check stent position with angiography before implantation.
Fractures were seen in at least three stents (7%). In one patient, the fracture was associated with a small vessel dissection, while in the two other cases the fracture was not associated with vessel obstruction, vessel damage or loss of stent integrity. Proper diagnosis of fractures requires fluoroscopic analysis, which was only done during follow-up in the patients who had repeat catheterisation. This means that additional type I fractures may have been missed. However, no type II or type III fractures were detected8. Stent fractures are usually rare in locations other than right ventricular outflow tracts. Although our study is very small, our incidence of stent fracture seems to be higher than the one reported with other stents9. This might be a correlate of high flexibility, open-cell design and limited number of struts. Because of the stent fragility, we would not recommend the use of this stent in infants with tetralogy of Fallot to open up very stenotic right ventricular outflow tracts.
Our study has several limitations. It is a retrospective study. It includes a broad spectrum of diseases and lesions. No precise haemodynamic or angiographic parameters could be analysed and compared to show haemodynamic and angiographic efficacy. Despite these limitations, our study gives a good overview of the possible applications, benefits and limits of the VALEO® stent in children.
Conclusions
We summarise and analyse our initial experience with a stent originally not designed for paediatric cardiac use. So far, it is the largest study analysing the use of the VALEO® stent in congenital heart disease. In our hands, the stent has been useful because of its low profile, its flexibility, the open-cell design and the possibility to redilate the stent further. We have, however, limited its use to “low-pressure” lesions (such as arterial ducts) or predilated “higher-pressure” lesions. It is not a robust stent and fractures do occur, probably at a higher incidence than with the other available stents. Because of the open-cell design it is difficult to diagnose these strut fractures, and longer follow-up is required. Stent dislodgement is possible and we strongly discourage advancing it without a long sheath. Longer follow-up is required.
| Impact on daily practice Stent placement in young and small children is a challenge. Stents don’t grow and the material is not perfectly adapted to small vessels or small hearts. Once a stent is placed in a growing child we know that further interventions will be required to match the growth. Stents such as the VALEO stent have a low profile and are redilatable to large diameters. Their implantation is easy and safe even in very small children. These arguments allow us to consider their use to enlarge vessels in small children, whenever other therapies are not suitable. |
Conflict of interest statement
The authors have no conflicts of interest to declare.

