Abstract
Aims: The lack of a specifically designed stent for the arterial duct has led to the off-label use of stents during hybrid palliation of hypoplastic left heart syndrome (HLHS). We evaluated the implantation and performance of a specially designed self-expanding stent in hybrid palliation of HLHS.
Methods and results: We implanted 39 sinus-SuperFlex (SSF) stents in 24 patients at a median age of seven days (range 2-27 days) and median weight of 2.85 kg (range 1.3-3.8 kg). A single stent was implanted in nine patients and two overlapping stents in 15 patients. There was one intraoperative death, not related to ductal stenting and one additional in-hospital death. During median follow-up of 137 days (range 38-522 days), nine patients required 11 interstage interventions. Four patients have undergone a biventricular repair, 11 have undergone the next stage of univentricular palliation and seven are awaiting a second-stage palliation.
Conclusions: The SSF stent provides effective maintenance of ductal patency in patients undergoing hybrid palliation of HLHS and its variants. It conforms to the ductal anatomy but the lack of stents longer than 20 mm has required overlapping stents in longer ducts. This has not been of haemodynamic consequence.
Abbreviations
AA: aortic atresia
AS: aortic stenosis
DNI: ductal narrowing index
HF: hemi-Fontan
HLHS: hypoplastic left heart syndrome
IAS: interatrial septum
LV: left ventricle
MA: mitral atresia
NW: Norwood
SSF: sinus-SuperFlex-DS
Introduction
The hybrid approach for the management of hypoplastic left heart syndrome (HLHS), consisting of bilateral pulmonary artery banding and ductal stenting, was initially introduced in the 1990s1,2. It has gained popularity and is currently being applied in a growing number of clinical settings. In many centres, it is offered as an alternative to initial palliation with the Norwood (NW) operation, with promising early and midterm results3-5. It has been performed as a bridge to heart transplant and as a bridge to the NW operation in unstable or high-risk patients6-9. There are variations in approach with some units opting to stage the procedure –starting with bilateral pulmonary artery (PA) band placement, then placing a stent in the ductus arteriosus percutaneously or via sternotomy several days later. Other units opt for concomitant hybrid stent placement at the same time as PA banding3,4.
In the early clinical experience, ductal stenting was performed using balloon-expandable stents1,2,4. These relatively stiff stents do not conform to the variable curvature of the duct and require significant radial force to expand them within the duct. Until recently, the lack of specially designed equipment has led to the off-label use of stents designed for other purposes3-6,10.
The sinus-SuperFlex-DS (OptiMed, Ettlingen, Germany) is a self-expanding, nitinol stent, specially designed to conform to the arterial duct in the HLHS. The aim of our study was to evaluate the implantation technique and midterm performance of this stent in the hybrid palliation of patients with HLHS and its variants.
Materials and methods
A retrospective data review was performed analysing patient characteristics, technical details and midterm follow-up of all neonates with HLHS or its variants who underwent hybrid implantation of SSF ductal stents at Evelina London Children’s Hospital. The study was conducted as an audit/quality improvement project and was approved by the institutional audit board. The need for individual consent for data collection was waived. Each patient’s initial management strategy had been decided following review at a multidisciplinary meeting.
Patients
From October 2012 to February 2014, twenty-four consecutive neonates, 10 (42%) with typical HLHS and 14 (58%) with various other forms of left heart obstruction, underwent SSF ductal stenting as part of a hybrid procedure. Median age at palliation was seven days (range two to 27 days) and the median weight 2.85 kg (range 1.3-3.8 kg). Patient characteristics and additional diagnoses are shown in Table 1 and Table 2. Diagnoses were established antenatally in 17 patients (71%). Five (21%) patients had additional comorbidities: duodenal atresia (two), coloboma and ophthalmic cyst (one), cleft palate (one) and CHARGE syndrome (one). The indication for hybrid palliation was weight <2.5 kg in seven (29%) patients, the potential for future biventricular repair in five (21%), complex anatomy in four (17%), poor right ventricular function with severe tricuspid valve regurgitation in two (8%), and a combination of the above in two patients (8%). In four patients (17%), both the standard NW and the hybrid procedures seemed equally applicable but the attending physicians’ choice was for a hybrid procedure. For statistical analysis, we divided our cohort into two groups, according to the number of implanted stents: Group I (single stent) and Group II (two overlapping stents).
During the same period, 12 patients underwent the standard NW I procedure in our institution. Prior to October 2012, 41 patients had undergone implantation of balloon-expandable stents for hybrid palliation in our unit.
Hybrid procedure
All interventions were performed in a biplane cardiac catheterisation laboratory under general anaesthesia, with cardiopulmonary bypass available in the adjacent cardiac operating theatre. After a median sternotomy, bilateral pulmonary artery bands were placed as previously described4,10,11. Prostin infusions were stopped during the initial surgical dissection. A purse-string suture was placed on the main pulmonary artery just above the pulmonary valve, and the tip of a short 6 Fr sheath was introduced. Angiography was performed by hand injection in the left lateral projection to delineate and measure the ductus. Minimal and maximal diameters as well as the length of the duct, from the origin of the left pulmonary artery to the descending aorta beyond the isthmus, were measured. A 0.018” guidewire was inserted through the duct into the descending aorta. An SSF stent was chosen that was 1 to 2 mm larger than the maximal ductal diameter and long enough to cover the whole length of the duct, from the origin of the LPA to just beyond the take-off of the left subclavian artery. If the duct was longer than the longest available stent, two overlapping stents were implanted, the first being deployed distally. Final angiography was performed to confirm stent position.
In order to confirm the capability of this stent to expand discrete narrowings within the duct, we produced a ductal narrowing index (DNI), measuring the ratio of the minimum to the maximum diameter of an individual duct before and after stent implantation.
The stent
The SSF stent is designed specifically for ductal stenting in HLHS. It is a self-expanding, open-cell stent, laser cut from a single piece of nitinol with no joints or welds. There are three stent diameters (7, 8 and 9 mm) and four lengths. The 7 mm stents are available in 15 and 18 mm lengths, the 8 mm in 12, 15, 18 and 20 mm lengths, and the 9 mm stent is available only in the 20 mm length. The 85 cm-long pre-loaded stent delivery system can pass through a 4 Fr sheath and accommodates a 0.018” guidewire.
Statistical analysis
All data analyses were performed using GraphPad InStat® software (GraphPad Software, Inc., San Diego, CA, USA). Data are presented as frequency, median with range, or mean±standard deviation, as appropriate. Continuous variables were analysed using a Mann-Whitney and Student’s t-test. Dichotomous and categorical variables were compared with Fisher’s exact and χ2 tests. Paired data were examined using paired two-tailed t-tests. The level of statistical significance was set at p≤0.05.
Results
DUCTAL CHARACTERISTICS
In the majority of patients (58%, n=14), the narrowest segment of the duct was at the pulmonary end. The smallest diameter was at the mid portion in five patients and at the aortic end in five patients. The minimum ductal diameters ranged from 1.8 to 7.1 mm (mean 5.1±1.3 mm), and the maximum diameters from 4.3 to 9.2 mm (mean 6.9±1.2 mm). The ductal length ranged from 10 to 25 mm (mean 17.4±3.7 mm). The DNI, calculated to characterise the severity of discrete ductal constrictions, ranged from 0.4 to 0.97 (mean 0.74±0.16).
STENT DEPLOYMENT
Thirty-nine SSF stents were deployed via a hybrid pulmonary artery approach in 24 patients, resulting in a significant increase of the minimum ductal diameter from 5.1±1.3 mm to 6.5±1.1 mm (p<0.001). The maximum ductal diameter also increased from 6.9±1.2 mm to 7.8±0.75 mm (p<0.001). The DNI increased significantly from 0.74±0.16 to 0.83±0.1 (p<0.008). The mean duration of the hybrid procedure (including pulmonary artery banding) was 109.2±54.7 min, the mean fluoroscopy time was 5.6±7.8 min, and the mean radiation dose was 23.3±20.5 cGym2.
A single stent was implanted in nine patients (Group I) and two overlapping stents in the remaining 15 (Group II). Within Group II patients, an initial ductal length measurement of more than 20 mm in seven patients indicated prospectively that a second stent would be required. In the remaining eight patients, the ductal length suggested that a single stent would be adequate; however, slight stent migration during deployment led to implantation of a second overlapping stent. In five patients the migration was distal and in three proximal. There was no statistical difference in age, weight, ductal diameter and length, as well as fluoroscopy and procedural times between Group I and Group II (Table 3). Group II received a higher radiation dose (p<0.007) and more frequently (three patients vs. one patient in Group I) required immediate post-dilation of the stents (p=0.57). The maximum pulsed-wave Doppler velocities in the stents were not significantly different on the first post-intervention or the latest follow-up echocardiograms. One patient in each group presented with retrograde aortic arch obstruction and ductal stent redilation was required in a single patient in each group.
There was no significant difference in the final minimal ductal diameter between those who started off with a significant narrowing and those with more uniform ducts (mean 6.3±1.3 mm vs. 7.0±0.8 mm, p=0.18).
ADDITIONAL INTERVENTIONS AT INITIAL PROCEDURE
One patient (4%) required ductal predilation before stent deployment and four (17%) required balloon dilation of the ductal stents immediately after deployment (Figure 1). None of these patients required further reinterventions on the duct. Four other patients underwent six additional interventions at the same procedure, including balloon atrial septostomy (four), atrial septal stent implantation (one) and aortic valve balloon dilation (one).
Figure 1. Implantation of two SSF stents with subsequent balloon dilatation in a 25 mm-long angulated duct. The wire tracks from the pulmonary artery sheath through the duct and into the descending aorta. A) Positioning of the first 9×20 mm stent in the distal duct. B) After deployment of second 9×20 mm stent into the proximal portion, both stents conformed to the shape of the duct with a kink (black arrow) in the mid segment. C) Inflation of a 9×20 mm Tyshak II balloon. D) Final angiography showing smooth passage of contrast to the descending aorta.
COMPLICATIONS
There was one procedural death, not related to ductal stenting. A 1.3 kg premature patient with critical aortic stenosis and borderline left ventricle underwent successful ductal stenting and aortic balloon valvoplasty. He had become haemodynamically unstable during pulmonary artery band placement. An adequate balloon atrial septostomy was performed following a failed attempt to stent his atrial septum; however, he continued to deteriorate and died during the procedure.
Minor complications occurred in two patients. Transient ST depression without haemodynamic compromise occurred in one patient after stent deployment, which resolved within a few minutes. Another patient had non-sustained ventricular tachycardia after balloon dilation of the stent.
OUTCOMES
The 23 survivors had immediate chest closure before transfer to the paediatric intensive care unit. Median ventilation time and intensive care stay were one day (range 1-4 days) and three days (range 2-21 days), respectively. The median hospital stay was nine days (range five to 108 days).
The mean pulsed-wave Doppler velocity through the stented duct on the day of the procedure was 1.2±0.4 m/s and all the patients had unobstructed retrograde aortic arch flow.
The survival to discharge from the hospital was 92% (22/24). There was one in-hospital death three days after the procedure. This patient, with HLHS, was extubated one day after successful hybrid palliation. Routine echocardiography on day three confirmed good ventricular function with a maximal Doppler velocity through the duct of 1.2 m/s and unobstructed retrograde aortic arch flow. She had an unexpected and profound deterioration with apnoeic seizures and subsequent bradycardia. Despite immediate resuscitation, she died. The autopsy did not show any concerns with the surgical anatomy and did not give a clear cause for the sudden death.
FOLLOW-UP
During a median follow-up of 137 days (range 38-522 days), the overall survival was 20/24 (83%) patients. Two (8%) patients died before discharge from the hospital as previously described (Figure 2). Six (25%) had a NW operation at a median age of 81 days (range 65-93 days), of whom three proceeded to hemi-Fontan (HF), two await the next stage surgery and one patient died in hospital. Five patients (21%) had a combined NW and HF at a median age of 115 days (range 80-141 days) with one in-hospital death. Four patients (17%) underwent a biventricular repair at a median age of 101 days (range 27-163 days). In all the patients, stents were removed easily at surgery, with no need for extended reconstruction of the previously stented aorta. Seven patients (29%) are awaiting the next stage of univentricular palliation.
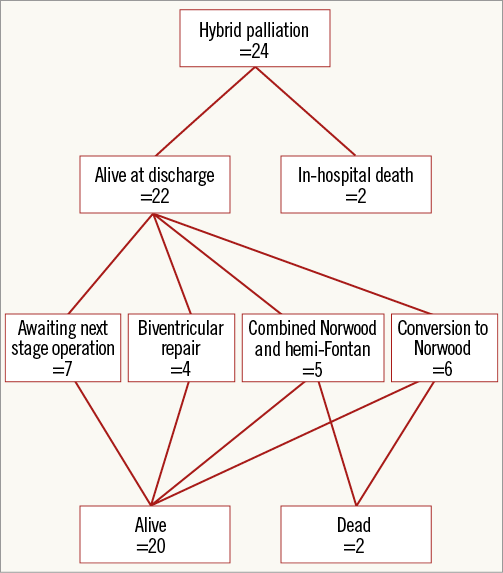
Figure 2. Outcomes after hybrid bilateral pulmonary artery banding and ductal stenting with the sinus-SuperFlex-DS stent.
Nine patients (41%) required 11 interstage interventions. Three patients underwent balloon atrial septostomy and three had atrial septal stent implantation, one via a hybrid approach12, one patient underwent aortic valve balloon dilation and four (18%) required reinterventions related to the previously implanted ductal stents.
One patient with aortic and mitral atresia and one with critical aortic stenosis and borderline left ventricle presented with an increased retrograde aortic arch velocity during planned follow-up, 50 and 114 days after the initial intervention. In both cases, implantation of 5×12 mm Liberté™ stents (one in one patient, two in the other) (Boston Scientific, Marlborough, MA, USA) resulted in improved haemodynamics and a reduction in the retrograde aortic arch Doppler velocity. Neither patient required further intervention and they underwent successful subsequent surgery, one with a combined NW procedure and HF and the second with a biventricular repair.
During follow-up, the mean ductal Doppler velocity increased from 1.2±0.4 m/s to 2.4±0.7 m/s (p<0.0001). Two patients had interventions for ductal stent obstruction, associated with decreased ventricular function 42 and 72 days, respectively, after the hybrid palliation. In the first, balloon dilation of the ductal stent was performed with a 9×20 mm Tyshak® balloon (NuMED, Inc., Hopkinton, NY, USA) and the second patient had a 7×18 mm PALMAZ® BLUE® (Cordis Europe N.V., Roden, The Netherlands) stent implanted within the original SSF stent (Figure 3). Both had improved ventricular function and Doppler velocity. One patient underwent a successful combined NW and HF operation 10 weeks later. The second is now 18 weeks old and awaiting their next operation.
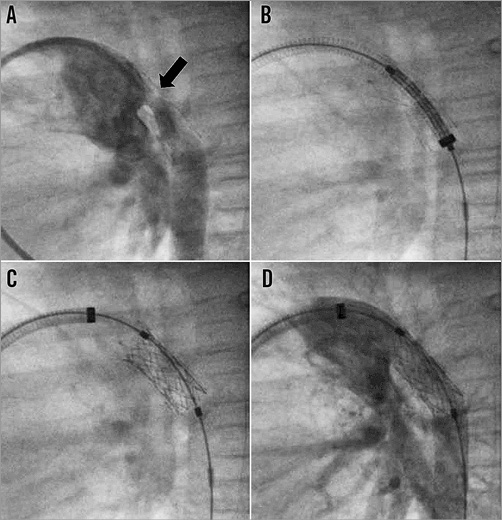
Figure 3. Reintervention for ductal stent obstruction. A) Prominent neointimal proliferation (black arrow) resulting in significant lumen narrowing of previously implanted self-expanding stent to 2 mm. B) Positioning of a balloon-expandable 7×18 mm PALMAZ BLUE stent through a 6 Fr long sheath. C) PALMAZ BLUE stent implanted within sinus-SuperFlex-DS stent. D) Final angiogram showing widely patent stents with unobstructed contrast flow to the descending aorta.
Discussion
We describe our experience with the most recent cohort of patients, who underwent hybrid ductal stenting with the first self-expanding stent specially designed for the arterial duct. Although self-expanding stents have been used for many years in the palliation of HLHS3-5,13, reports of operators systematically using them in this context are scarce14. To the best of our knowledge, this is the first description of an exclusive use of the SSF stents.
The design features of the nitinol SSF stent allow it to conform to the irregular and variable ductal anatomy found in HLHS. The radial force exerted during the deployment of a self-expanding stent is lower than that applied during balloon-expandable stent deployment15,16. This may result in less trauma to the wall of the arterial duct. Furthermore, the flow dynamics produced by a flexible, conformable self-expanding stent may result in better haemodynamics than those achievable with a more rigid balloon-expandable stent17. Figure 4 illustrates an aspect of the mechanical load-bearing properties of self-expanding compared with balloon-expandable stents. Other authors have limited the use of self-expanding stents to wide, unobstructed ducts4. Our population included patients with many ductal morphologies, including significant constrictions, and our data suggest that this particular stent has enough radial force to maintain ductal patency and deal with most ductal narrowings. In a minority of cases, we elected to balloon dilate any residual narrowings, which we felt may encourage intimal ingrowth or poor haemodynamics, and found that the SSF stent maintained these dilated diameters well.
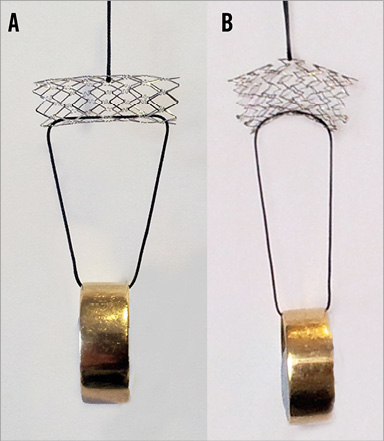
Figure 4. Qualitative demonstration of the flexibility of a balloon-expandable and self-expanding stent. Example of a balloon-expandable stent – 9×22 mm PALMAZ® GENESIS® (Cordis) (A) and a self-expanding stent – 9×20 mm sinus-SuperFlex-DS (B). The stents were vertically suspended with an identical load to illustrate the difference in flexibility after expansion.
Many reports have emphasised the importance of covering the whole ductal length to avoid recurrent obstruction after prostaglandin cessation14,18. As the SSF stent is available in a limited range of lengths up to 20 mm, two overlapping stents have been required for longer ducts. We have not observed any haemodynamic consequences of overlapping stents, either immediately or during follow-up. However, this group of patients needed more frequent post-dilation.
Despite the anti-jump mechanism described in the company’s product information literature, we have noted movement of the stent on deployment in keeping with other self-expanding stents. On eight occasions a second stent was needed because of the movement of the stent, whereas the initial measurements suggested that a single stent would have been adequate. This may reflect a learning curve in deployment technique. Availability of a longer stent may allow better tolerance of minor stent movement on deployment as well as of measurement errors in patients with longer ducts.
Two minor procedural complications occurred in patients with overlapping stents. These complications were self-limiting and could not be causally related to stent deployment or the presence of a second stent.
In our cohort, two (9%) of the 22 surviving patients required reintervention because of reduced antegrade flow through the ductal stent. This was less frequent than the incidence (18%) reported by Galantowicz et al and Nadorlik et al3,19 and similar (11%) to that reported by Baba et al14. In our experience, immediate low-pressure balloon dilation of the stent, in those in whom there was a residual waist, has avoided the need for further reintervention. Those patients who required redilation of the ductal stent during follow-up had moderate residual waists after the initial stenting, which were not dilated immediately. One required balloon dilation only, whilst the other (who had developed significant intimal in-growth shown in Figure 3) required placement of a balloon-expandable stent within the SSF stent.
Retrograde aortic arch obstruction, especially in patients without forward flow in the ascending aorta, may lead to severe complications, such as myocardial or neurological ischaemia. It has been reported to occur in up to 29% of patients20,21. We observed a similar rate of retrograde aortic arch obstruction (8%) to Baba et al14. We relieved retrograde obstruction with implantation of a coronary stent via a femoral artery approach. This did not have a negative influence on surgical removal of the stents and arch reconstruction at subsequent surgery.
In the 15 patients who have undergone the next stage of surgical palliation, we have found that the stents, irrespective of whether single or overlapping, are easy to remove. There was no need for additional, extensive surgical dissection and repair, qualitatively different from our previous experience with balloon-expandable stents.
Hospital survival in our group was 92% with no further interstage mortality. This may be influenced by a change in our approach to the palliation of single ventricle patients, with the introduction of a NW stage I operation six to eight weeks after the hybrid intervention in selected patients, with the HF operation being performed at the age of about six months22.
Limitations
This study is retrospective, with too small a cohort for strong statistical analysis, and the experience is limited to a single institution. Although most of the patients discharged after the initial procedure have already had their stent surgically removed, it is not possible to predict any potential changes of the mechanical properties of the reconstructed aorta in this cohort. In addition, surgical treatment was carried out relatively early after the stent implantation, so it is impossible to define the behaviour of the SSF stents and the aorta if left in place for a longer period of time, as can be the case in those patients planned for biventricular repair.
Conclusions
The sinus-SuperFlex-DS provides effective maintenance of ductal patency in patients undergoing hybrid palliation of hypoplastic left heart syndrome and its variants. It conforms well to the shape of the duct. The lack of stents longer than 20 mm requires overlapping stents in longer ducts. This has not been of haemodynamic consequence. Further studies comparing the SSF stent with other self-expanding and balloon-expandable stents are warranted.
| Impact on daily practice Developments in interventional congenital cardiology are plagued by a lack of equipment specifically designed for our purposes. The use of relatively stiff balloon-expandable coronary or biliary stents in the neonatal ductus arteriosus raises practical and physiological concerns. The sinus-SuperFlex stent has been specifically designed to accommodate the soft curvature of the ductus with the hope that it will allow more physiological flow as well as decreasing complication rates and allowing for easier surgical explantation at the time of arch reconstruction. This stent offers distinct, attractive characteristics when considering stent choice in hybrid palliation of hypoplastic left heart syndrome and its variants. |
Conflict of interest statement
The authors have no conflicts of interest to declare.

