
Abstract
Aims: The aim of this study was to assess the efficacy and safety of percutaneous reintervention in patients who underwent aortic coarctation stenting at an early age.
Methods and results: From 1993 to 2018, 177 patients with aortic coarctation were treated with stent implantation at our centre; 33 of them were treated at less than 12 years of age and required reintervention because of their rate of growth. The average age of the patients at the first and second procedure was 6±3 years and 19±7 years, respectively. At the reintervention procedure, 15 (45%) patients were treated with balloon re-expansion, and 18 (55%) were treated with re-stenting. Success was obtained in 30 patients (91%). The gradient across the coarctation changed from 22±10 mmHg to 6±6 mmHg, while the minimal lumen diameter increased from 9±6 mm to 15±3 mm. There were eight occlusions of the femoral artery (after the first procedure), and two covered stents were needed because of femoral bleeding. The mean follow-up time after the second procedure was 5±4 years. A third procedure was required only in three patients (9%).
Conclusions: Patients with aortic coarctation treated with stent placement at an early age can be successfully re-treated after the completion of their somatic growth.
Introduction
European and American guidelines1,2,3 recommend an intervention in all patients with significant aortic coarctation (class I, level of evidence C). Since the initial descriptions of stent placement for aortic coarctation4,5, this approach has become the preferred treatment for adults in many centres. Several excellent registries have demonstrated the safety and efficacy of bare metal6,7 or covered stents8 for the treatment of aortic coarctation. The same guidelines also support this strategy in adults with native aortic coarctation and appropriate anatomy1,2. However, the aforementioned recommendations have not been extended to growing children or young adults without a definitive aortic size. The reason is the limited information available with regard to stent re-dilation in patients with aortic coarctation. Although some previous studies have reported a high rate of procedural success after balloon re-dilation9, the time interval between stent implantation and re-dilation was short. In this context, the aim of our investigation was to assess the efficacy and safety of percutaneous reintervention in patients who underwent aortic coarctation stenting at an early age. The second procedure was scheduled several years after stent implantation when the aorta reached its near definitive size. For this purpose, we analysed a series of patients with aortic coarctation treated with stent placement who needed balloon re-dilation or re-stenting because of their somatic growth.
Methods
PATIENTS
From 1993 to 2018, 177 patients with aortic coarctation were treated with stent implantation at our centre. For this particular study, we selected 33 patients treated with stent implantation when they were younger than 12 years old and who required reintervention because of their rate of growth. We did not include any adults or patients less than 12 years old without reintervention who were awaiting their definitive aortic size in this analysis. The patient flow chart and study design are presented in Figure 1.
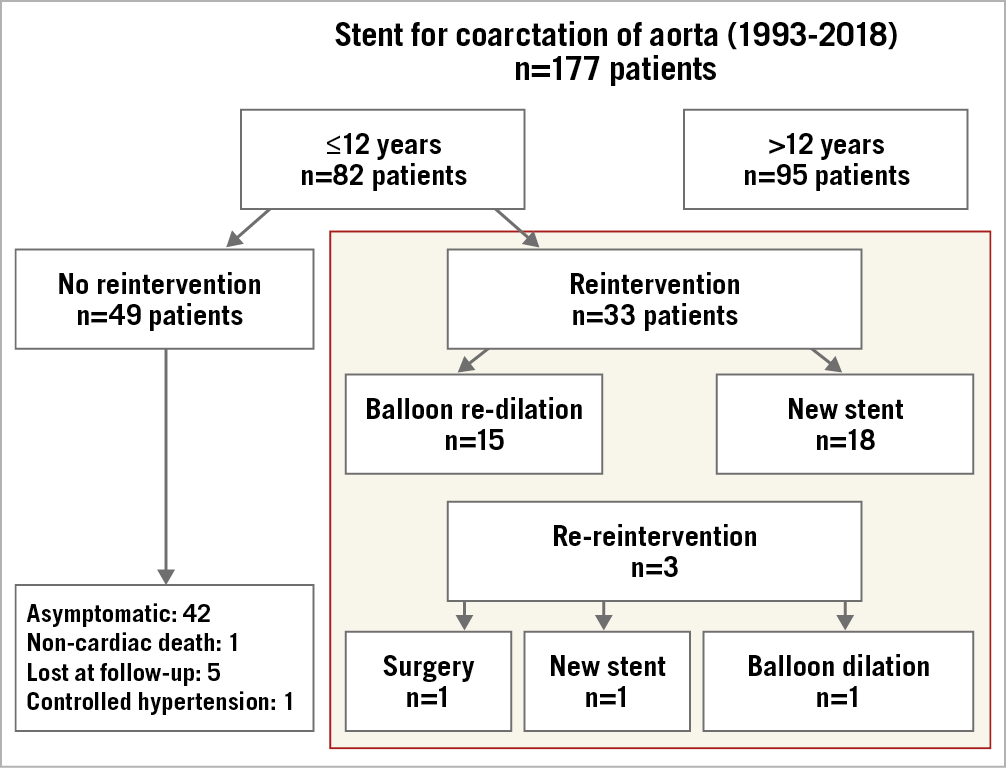
Figure 1. Patient flow chart.
STUDY ENDPOINTS AND DEFINITIONS
The primary endpoint of the study was to determine the immediate success and major complication rates of the reintervention procedure (re-dilation, re-stenting) several years after stent implantation at an early age in patients with aortic coarctation. The secondary endpoints were to determine the incidences of hypertension and major events during the follow-up period after the reintervention procedure. We defined primary success as a reduction in the gradient across the coarctation segment greater than 50% without major complications (including serious femoral access bleeding).
PROCEDURE
Two femoral or radial/femoral accesses were obtained. The simultaneous peak-to-peak pressure gradient across the coarctation segment was measured before and after treatment. Angiography at different projections was always performed to assess the anatomy of the aorta and the previously stented segment. Measurements and evaluation of fractures or recoils of the first stent were also carried out before the new treatment. Angiographic measurements were obtained at different levels of the aortogram on a 60° left anterior oblique (LAO) projection. The minimal lumen diameter at the coarctation level, the isthmus diameter at the level of the left subclavian artery, and the aortic diameter at the level of the diaphragm were also measured. The percentage of stenosis was calculated as (1- aortic diameter at the coarctation/aortic diameter at the diaphragm) × 100. After the diagnostic phase, the balloon size was selected according to the diameter of the aorta at the level of the diaphragm. The balloon type varied over 15-20 years. New balloon models were incorporated once they were available in our hospital. Initially, we used the OPTA® balloon (Cordis, Roden, the Netherlands). When NuMED balloons became available at our centre, the BIB® balloon (NuMED, Inc., Hopkinton, NY, USA) was used for stent deployment and a Z-MED™ or Mullins balloon (both NuMED, Inc.) for stent dilation. After stent deployment, an additional balloon inflation was performed in the case of non-stent apposition in the dilated aorta at the post-coarctation segment. Re-stenting was considered when a suboptimal result was obtained after balloon dilation or when multiple stent fractures occurred (Figure 2). A covered stent was selected based on the criteria of the operator, mainly used in cases of minimal aortic wall damage (Figure 3) or aneurysms (Figure 4). A covered Cheatham-Platinum (CP) stent (NuMED, Inc.) or a BeGraft stent (Bentley Innomed GmbH, Hechingen, Germany) was introduced through a 12-14 Fr sheath. Left radial or contralateral femoral access was used to monitor stent deployment through short contrast injections performed with a 4-6 Fr pigtail or multipurpose catheter. Beginning in 2011, the femoral puncture site was closed with a Prostar® (Abbott Vascular, Santa Clara, CA, USA) 10 Fr device (previously implanted before cannula insertion). Signed informed consent was always obtained before the procedure.
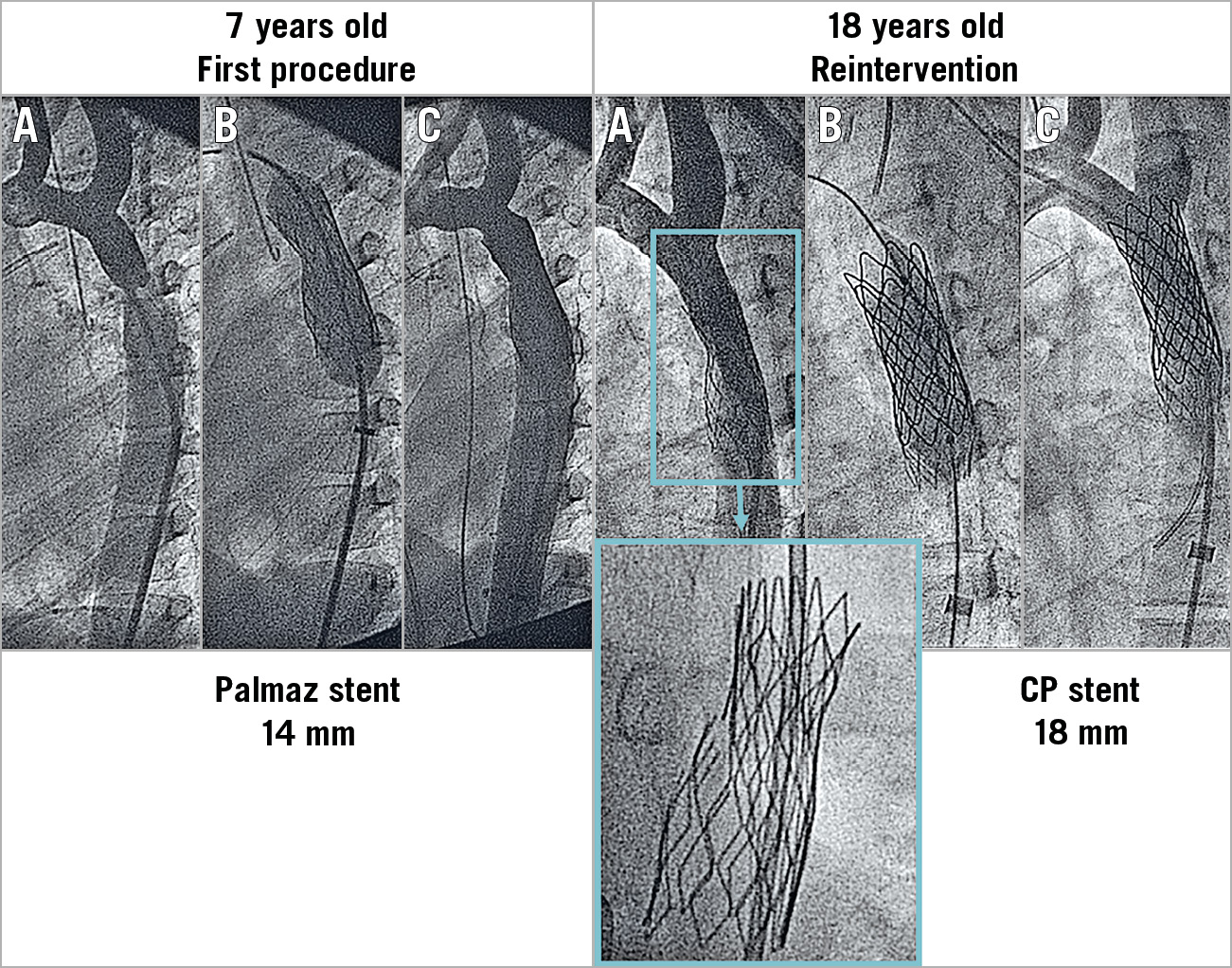
Figure 2. Example of a patient with aortic coarctation treated with stent placement at seven years of age. Re-stenting at 18 years of age. A) Baseline. B) Stenting. C) Post-stenting. The first Palmaz stent developed multiple strut fractures 11 years after the implantation.
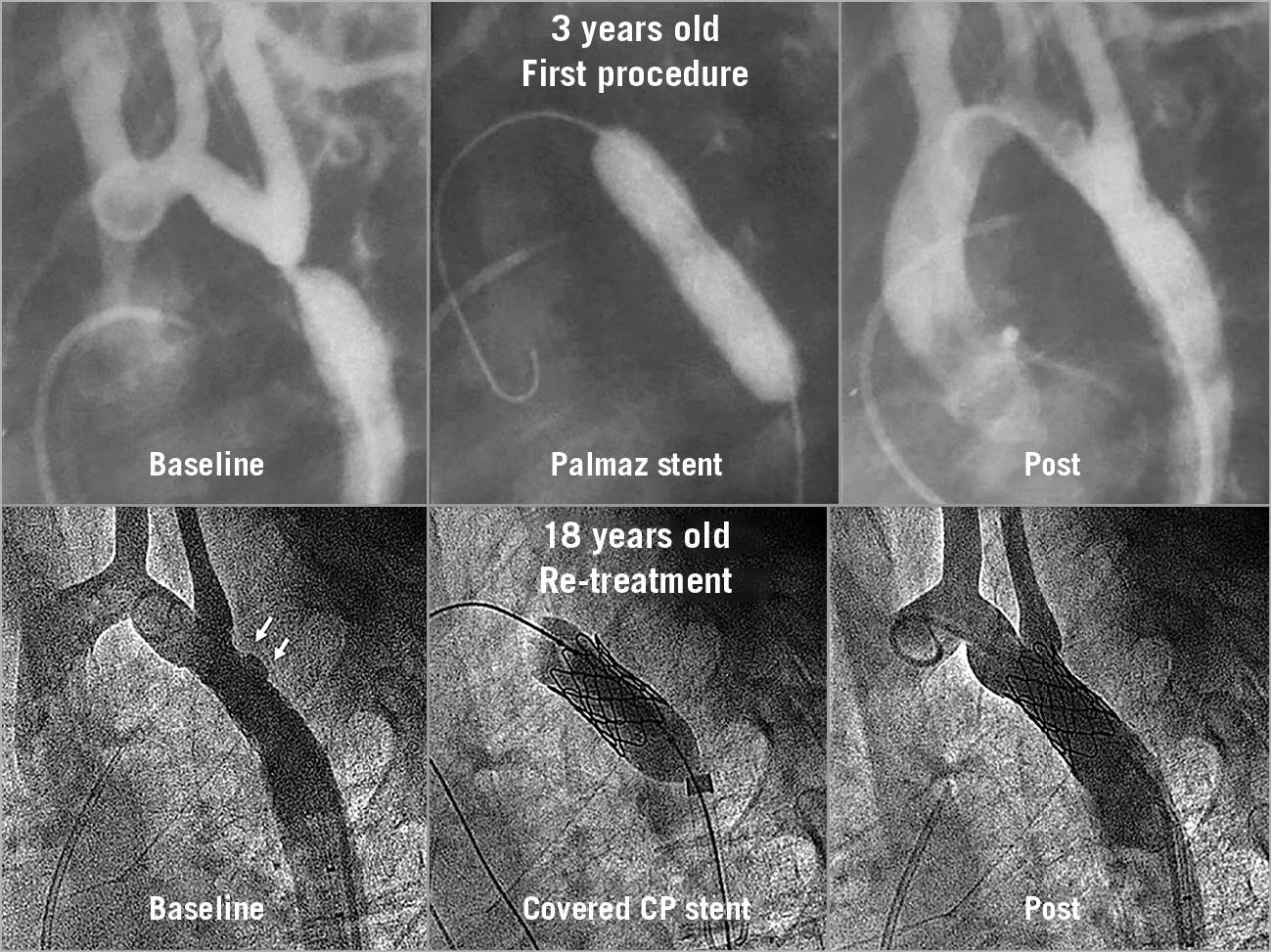
Figure 3. Example of a patient with aortic coarctation treated with stent placement at three years of age. Re-stenting at the age of 18 years. Small bulging area of the aorta outside the first stent (arrows) that was resolved with a second covered CP stent.
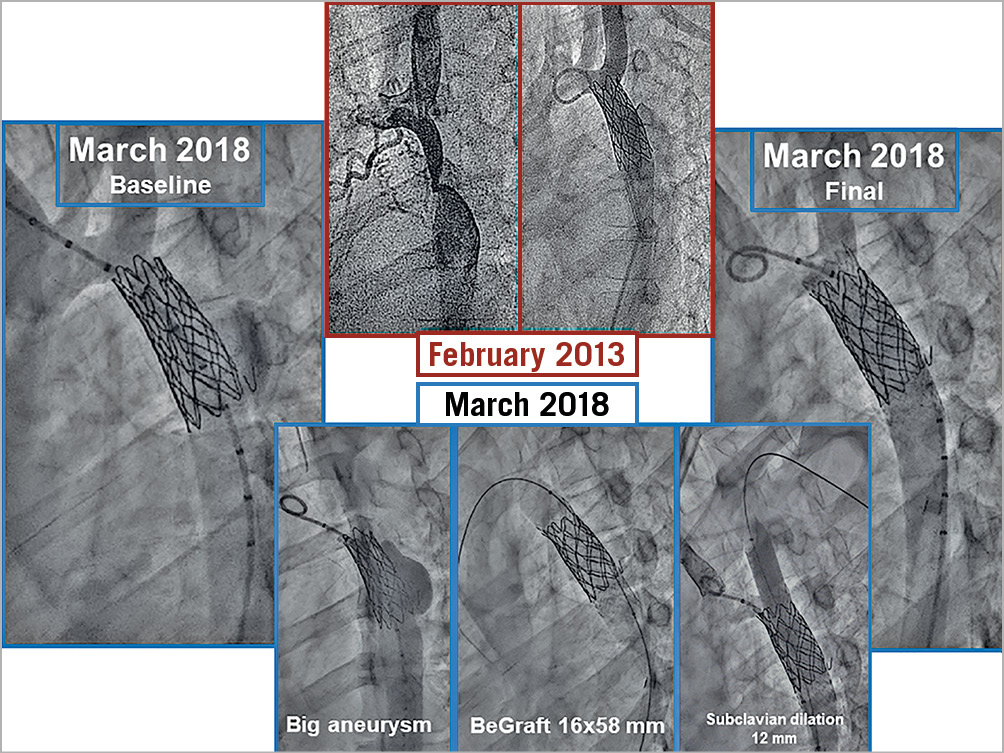
Figure 4. Example of a patient with an aneurysm after stenting at an early age. A covered CP stent used in the second procedure increased the aortic diameter and resolved the malformation.
FOLLOW-UP
Follow-up studies included telephone calls and scheduled clinical and transthoracic echocardiographic (TTecho) evaluations at six months, one year, and every subsequent year. A follow-up computed tomography (CT) scan was performed in all patients beginning in 2008 after the first stent was placed to detect the presence of aneurysms in the treated aortic segment. Additional cardiac catheterisation was recommended if an aneurysm or a significant gradient across the stented segment was present. Late major events, the incidence of hypertension and the need for medication were recorded. After the reintervention procedure, the patients were followed in the outpatient clinic, and serial X-ray pictures, TTecho and peripheral Doppler studies were obtained in all patients. CT studies were available in 13 patients.
STATISTICAL ANALYSIS
Quantitative data are expressed as the mean±standard deviation. Student-Fisher paired or unpaired t-tests were used to compare the means from the same patients or from different groups of patients. Differences between the proportions were studied using a chi-square test. All statistical analyses were performed using SPSS, Version 20.0.0 software (IBM Corp., Armonk, NY, USA). A value of p<0.05 was considered statistically significant.
Results
BASELINE AND PROCEDURAL DATA
The baseline clinical and angiographic data are shown in Table 1. Nine patients had associated malformations, and four had previous surgery of the aortic coarctation. The time interval between aortic stenting and reintervention was 13±5 years. During this period, the aorta grew 4 mm on average at the level of the isthmus below the level of the subclavian and 6 mm at the level of the diaphragm (Table 1). Asymptomatic occlusion of the femoral artery (as a result of the previous procedures) was observed in eight patients (24%). The procedural data are also summarised in Table 1. The majority of the patients (n=27, 82%) were treated with a Palmaz stent (Cordis, Cardinal Health, Milpitas, CA, USA) during the first procedure (Figure 3, Figure 5), while 15 patients (45%) were treated with balloon re-expansion during the second intervention (Figure 5). When a suboptimal result after balloon dilation or poor integrity of the first stent was observed (Figure 2), a second stent was implanted. Stent fractures were a frequent finding (n=15, 45%) after this long-term follow-up period (Figure 2); however, stent fractures were not associated with restenosis or aneurysm formation and facilitated expansion of the second stent. We could not identify any factors responsible for stent fractures. The time interval between the two procedures was similar between patients with and without these fractures (14±6 years vs 13±3 years; p: ns). The age at stent implantation was also similar between patients with and without fractures (7±4 years vs 5±4 years; p: ns). The kind of stent was also not a factor determining stent fractures: Palmaz 11 (40%) vs CP stent 4 (66%); p: ns.
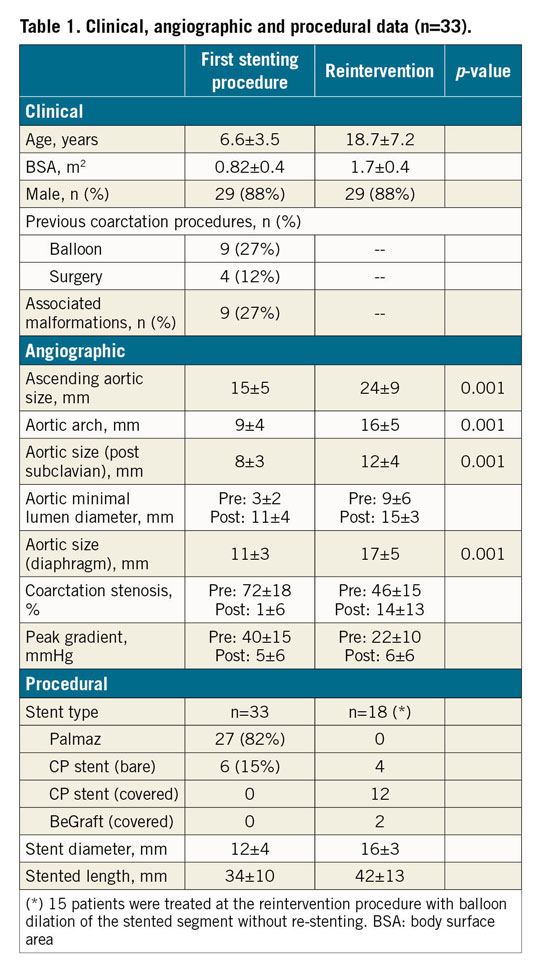
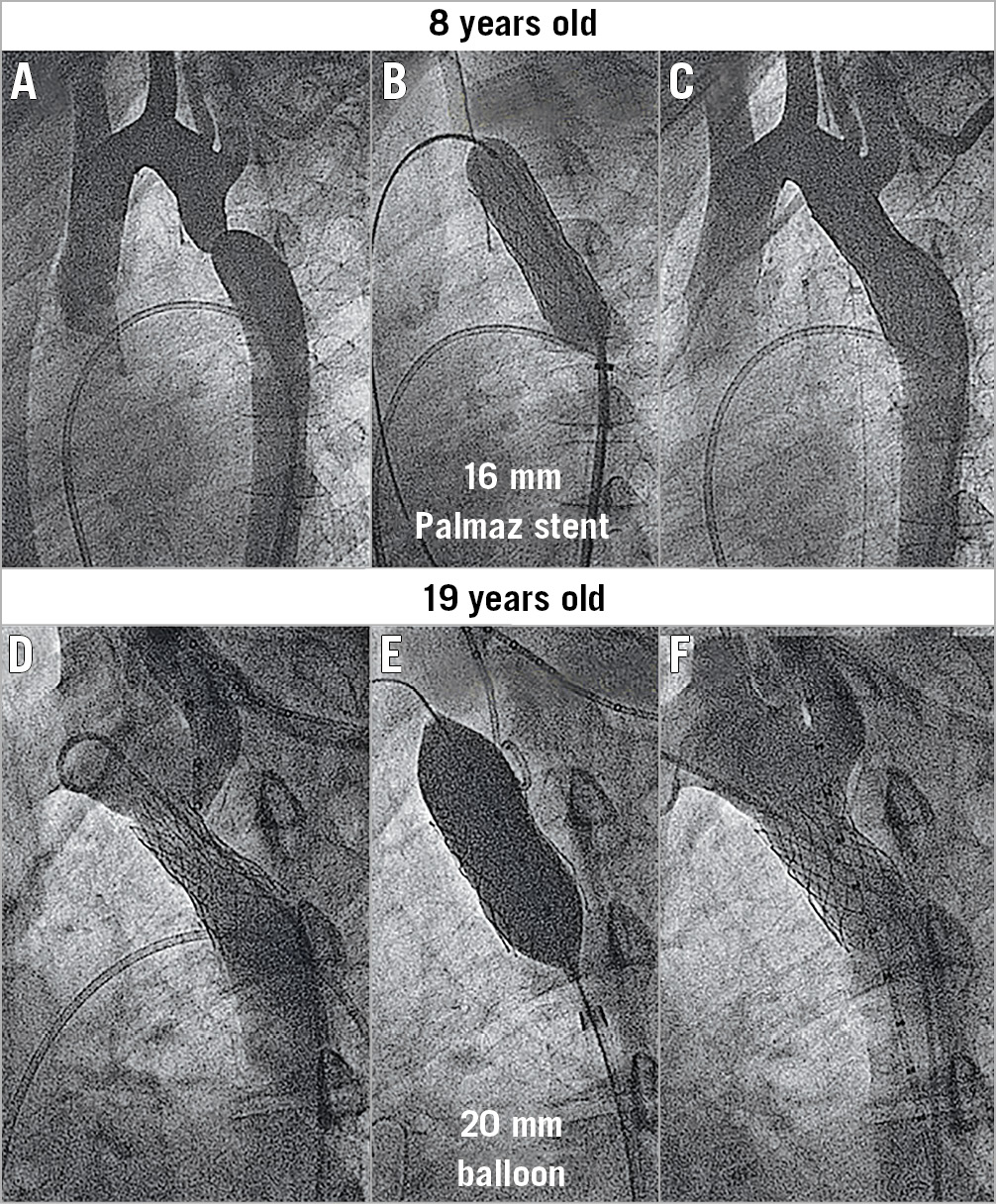
Figure 5. Example of a patient with aortic coarctation treated by stent placement at eight years of age and successful balloon dilation of the stent 11 years later. A) Baseline. B) Treatment. C) Post treatment.
PRIMARY ENDPOINT
Primary success was obtained in 30 patients (91%). An immediate reduction in the peak gradient across the coarctation was achieved in the majority of patients (Figure 6). The final residual gradient was 6±6 mmHg. In one patient without primary success, the stent remained unexpanded despite repeated dilations. In the remaining two patients, primary success was not obtained because of puncture site bleeding requiring covered stents.
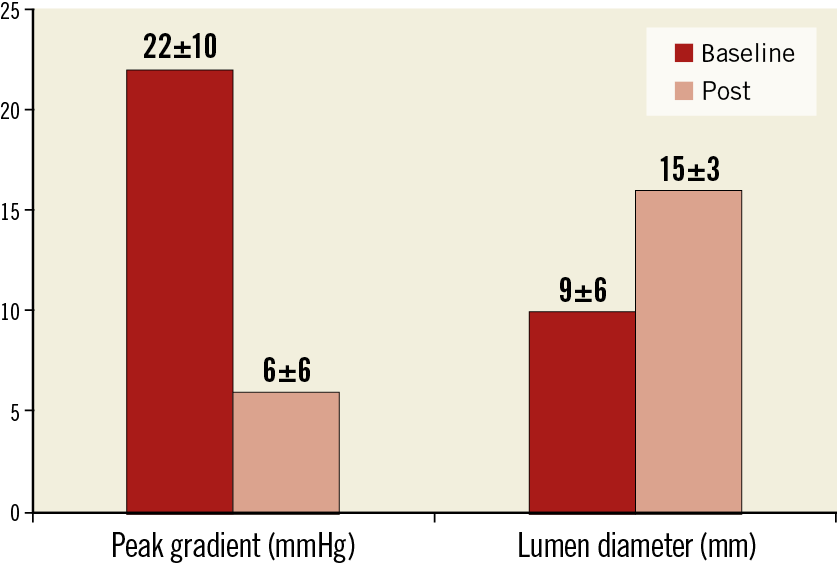
Figure 6. Changes in the peak gradient and lumen diameter after reintervention for coarctation.
ANGIOGRAPHIC RESULTS AND IN-HOSPITAL OUTCOME
The quantitative angiographic data are summarised in Table 1. The different aortic segments increased significantly in size between the two procedures due to somatic growth (Table 1). Nine patients presented some degree of aortic wall damage at the time of the reintervention procedure: six had limited aortic bulging (Figure 3), and three had small aneurysms (Figure 4). After coarctation reintervention, the minimal lumen diameter at the level of the coarctation changed from 9±6 mm to 15±3 mm (p<0.001), and the percentage of stenosis changed from 46±15% to 14±13% (p<0.001) (Figure 6).
During the reintervention procedure, two patients experienced significant groin bleeding requiring a covered stent at the puncture site. In both patients, the sheath used for aortic stenting was 14 Fr. A femoral covered stent was needed because of a failure in the puncture closure device. We preferred this percutaneous approach because it is faster than surgery and can be applied in the laboratory immediately after the diagnosis of the complication. Both patients are currently asymptomatic. The remaining patients were discharged two to three days after the procedure without any major complications.
SECONDARY ENDPOINTS AND CLINICAL FOLLOW-UP
The mean follow-up time after the second procedure was 5±4 years. A third procedure was required in three patients (9%) in whom the stent was implanted at less than two years of age (Figure 1). The first patient with suboptimal results after balloon dilation (two years after stenting) required cardiac surgery several months later because of stent underexpansion. We do not know the reason for this difficulty in the re-expansion of the stent, but the surgeon resected the stented segment and performed an end-to-end anastomosis. The second patient required balloon dilation and re-stenting at six years and 26 years, respectively, after the first procedure. In the third patient, re-stenting and high-pressure balloon dilation were performed at 14 years and 23 years, respectively, after the first procedure. Only one additional patient continued to have controlled hypertension (one drug) despite a successful reintervention procedure. The remaining 29 patients are currently asymptomatic and free of systemic hypertension after 5±4 years of follow-up. Aortic wall injuries were not identified after the second procedure in the group of patients who had follow-up CT scans (n=13). According to TTecho and Doppler studies, there were no cases of re-coarctation.
Discussion
In our study, we analysed the safety, efficacy and long-term outcomes of reintervention procedures (re-dilation, re-stenting) in children with aortic coarctation many years after the first stenting procedure. Our findings suggest that patients with aortic coarctation treated with stenting at an early age can undergo successful reintervention with balloon dilation or re-stenting after a mean time interval of 13 years, when somatic growth was complete. Any events at follow-up (9% of reinterventions) occurred only in patients who were younger than two years old when they were first treated.
STENTING THE AORTA IN GROWING CHILDREN
Stenting of aortic coarctation has not been recommended in growing children without a definitive aortic size10. The stent diameter obtained at an early age may be insufficient after completion of somatic growth, and a second procedure seems to be warranted. Re-expansion of the stent could be difficult; limited information is available about the second procedure that should be performed many years after the first aortic stent is placed. During recent years, some devices have been designed to be easily re-expanded even several years after implantation.
Ewert et al11 described the “growth stent” as a new transcatheter treatment for aortic coarctation from infancy to adulthood. The stent is made of two separate longitudinal halves that are connected with bioabsorbable sutures. Although this was an excellent proposal, there are no available data about long-term follow-up of patients who were treated with this method.
Another interesting device is the Valeo stent (Edwards Lifesciences, Irvine, CA, USA)12. This stent is available in different diameters (6-10 mm) and lengths (18-56 mm). The required sheath size is 6-7 Fr and can be expanded up to 20 mm. Based on these favourable stent properties, Kang et al13 successfully treated 14 children between two and eight years old with aortic coarctation. They only reported two stent re-dilation procedures performed four and five years after Valeo stent implantation. Therefore, information regarding the long-term outcomes of aortic stenting in growing children is only available for the Palmaz or CP stent, as described in this series. These devices have a significant probability of fractures and the possibility for re-expansion many years after the implantation.
STENT RE-DILATATION IN AORTIC COARCTATION
Reintervention for aortic coarctation after previous stent implantation performed many years previously has been considered a complex procedure14. Zanjani et al9 demonstrated the feasibility of stent re-dilatation in patients with aortic coarctation. The authors re-dilated stents in 22 patients, most of them previously treated with a Palmaz or CP stent. The primary success of the procedure was 93%, but the interval between stent implantation and re-dilation was short (median, 18 months). The reasons for failure were a lack of stent expansion (4%) or aneurysm formation after the procedure (4%). Thanopoulos et al15 re-dilated stents in 14 patients with growth-related re-coarctation (size mismatch between the stented segment and the proximal and distal aortic segments) five to six years after stent implantation. No complications were observed because of the re-dilation procedures. In an important observational study by the Congenital Cardiovascular Interventional Study Consortium (CCISC)16, 350 patients with aortic coarctation treated with different interventions were included. Stent implantation was performed in 217 patients, 44 of whom needed reintervention for different reasons one to three years after the first procedure. The authors did not specify how many reinterventions were performed in children or adults. The success rates, complication rates and follow-up outcomes of these second procedures were not reported. An additional small series with a short interval (one to three years) between stent implantation and reintervention supported the previously discussed good immediate results17,18,19,20,21. In our series the only complication observed during the re-dilation procedures was severe puncture-site bleeding in two patients. Both patients required covered stents. Although they remain asymptomatic, a close follow-up is needed in order to detect femoral stent fractures or restenosis.
THERAPEUTIC STRATEGIES IN GROWING CHILDREN
In infants and children younger than one year of age, surgery seems to be recommended for most cases of native aortic coarctation and balloon angioplasty for most cases of recurrent coarctation. Between the ages of one year and the time when the child reaches a weight of 15 kg (two to three years), treatment of coarctation must be individualised (balloon angioplasty or surgery) depending on the coarctation anatomy and the presence or absence of hypoplasia of the isthmus. According to our findings, older children (2 to 12 years old) could be treated with a first stenting procedure while accepting the need for a second procedure after completion of their somatic growth. The use of novel balloons and stent technology (e.g., a Valeo stent) with much smaller sheath sizes may significantly diminish the number of vascular incidents (Figure 7).
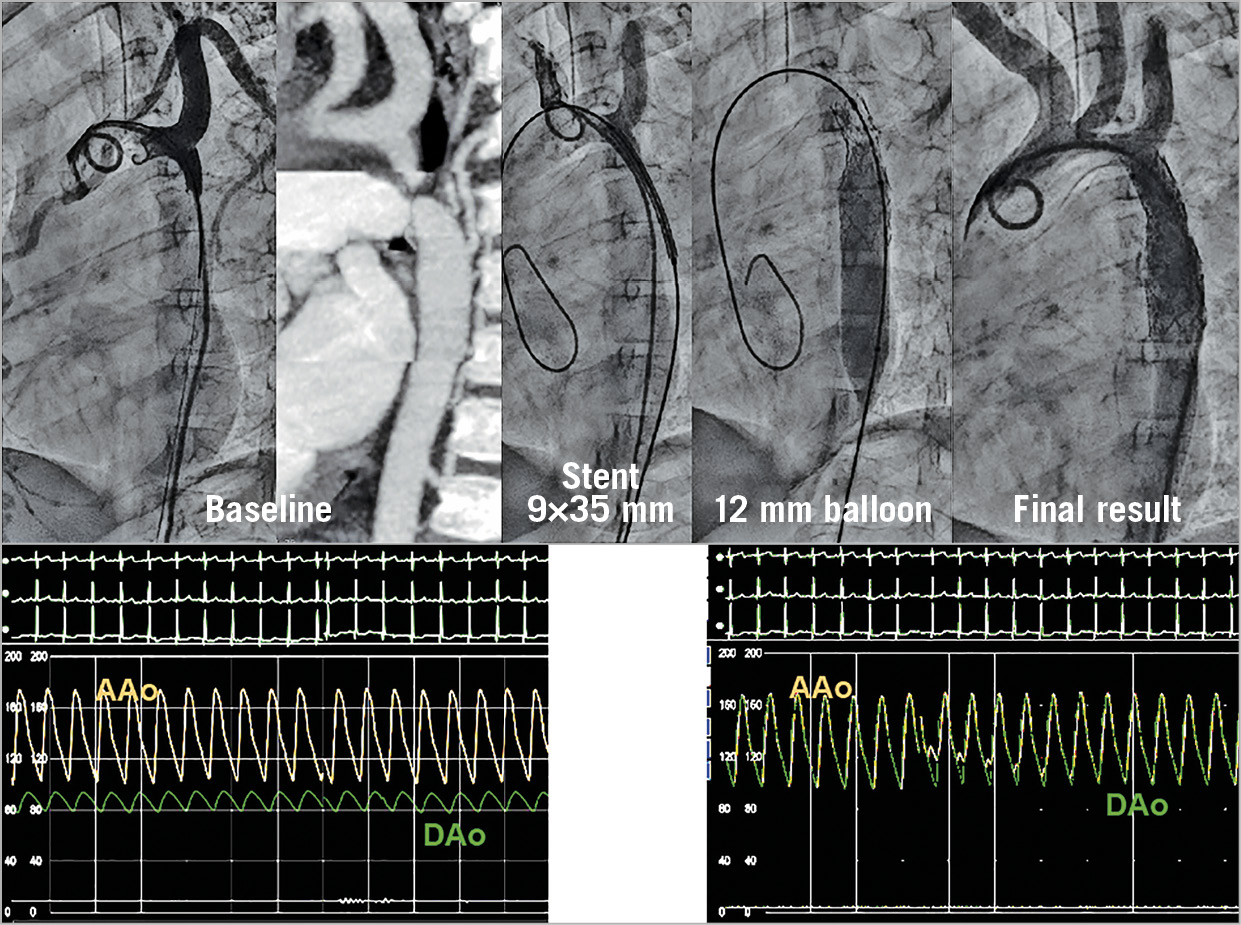
Figure 7. Example of a four-year-old patient with aortic coarctation treated with a Valeo stent. A 7 Fr sheath was used to introduce the stent.
Limitations
The main limitation of the study is the small sample size. Nonetheless, our series of stent re-dilation procedures performed in children with aortic coarctation is, to our knowledge, the most exhaustive study in the literature. Furthermore, this study had the longest reported follow-up period – 13 years between the first and second procedure and five additional years after the reintervention procedure.
Conclusions
Patients with aortic coarctation treated with stent placement at an early age can be successfully re-treated after the completion of their somatic growth.
|
Impact on daily practice Children with aortic coarctation treated with stenting at two to 12 years can undergo successful reintervention after the completion of their somatic growth. A strategy for early aortic stenting for aortic coarctation could be considered while accepting the need for a second procedure. |
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

