Abstract
The catheterisation laboratory today combines diagnosis and therapeutics, through various imaging modalities and a prolific list of interventional tools, led by balloons and stents. In this review, we focus primarily on advances in image-based coronary interventions. The X-ray images that are the primary modality for diagnosis and interventions are combined with novel tools for visualisation and display, including multi-imaging co-registration modalities with three- and four-dimensional presentations. Interpretation of the physiologic significance of coronary stenosis based on prior angiographic images is being explored and implemented. Major efforts to reduce X-ray exposure to the staff and the patients, using computer-based algorithms for image processing, and novel methods to limit the radiation spread are being explored. The use of artificial intelligence (AI) and machine learning for better patient care requires attention to universal methods for sharing and combining large data sets and for allowing interpretation and analysis of large cohorts of patients. Barriers to data sharing using integrated and universal protocols should be overcome to allow these methods to become widely applicable. Robotic catheterisation takes the physician away from the ionising radiation spot, enables coronary angioplasty and stenting without compromising safety, and may allow increased precision. Remote coronary procedures over the internet, that have been explored in virtual and animal studies and already applied to patients in a small pilot study, open possibilities for sharing experience across the world without travelling. Application of those technologies to neurovascular, and particularly stroke interventions, may be very timely in view of the need for expert neuro-interventionalists located mostly in central areas.
Introduction
The entire catheterisation laboratory environment is undergoing a major revolution. In recent years we have seen remarkable innovations leading to a marked improvement to our imaging modalities and methodologies for analysing and visualising cardiovascular structures. While X-ray hazards continue to pose a constant threat to medical personnel and patients, new modalities for reducing radiation exposure are constantly being developed. Computational and artificial intelligence (AI)-based interpretation of coronary images is now being explored and applied to our patients in both the research and clinical arenas. Robotic interventions, originally developed and implemented in the surgical arena, are now entering the catheterisation laboratory.
This review focuses on current and future trends in coronary interventions, with particular emphasis on the X-ray fluoroscopy environment, which is being enhanced by new imaging and visualisation methodologies that leverage machine learning and AI to help advanced patient diagnoses and therapeutics. Integration of physiological measurements into our clinical paradigm help tremendously in creating better decision-making tools for interventionalists. Since these new methodologies are being used within catheterisation laboratories and the harsh environment of radiation exposure, recent developments to reduce that exposure will be discussed. Recently developed robotics and automation for percutaneous coronary and neurovascular procedures aim to remove the operator from this hostile environment and increase procedural precision. These developments have opened up a new world of possibilities regarding remote procedures and partial or complete automation.
ADVANCED IMAGING TECHNOLOGIES AND VIRTUAL REALITY IN INTERVENTIONAL CARDIOLOGY
Cardiovascular imaging has evolved tremendously over the past two decades. Today, imaging is crucial in both diagnosis and as the primary guidance tool during interventions. The Central illustration lists the various imaging modalities that are used before, during, and following coronary and cardiac interventions with a few visual examples. Use of these technologies in various forms, as well as the overriding AI surveillance, are discussed below. The primary imaging modalities during coronary interventions are X-ray fluoroscopy and angiography. With the advances in digitisation of coronary angiography at the end of the 1990s, computer-assisted image analysis became feasible. This enabled objective measurement of coronary obstructions by quantitative coronary angiography. It was a breakthrough for routine diagnostic evaluations and for providing novel therapeutic methodologies of the coronary arteries1,2. Quantitative coronary angiography is now integral to every interventional laboratory.
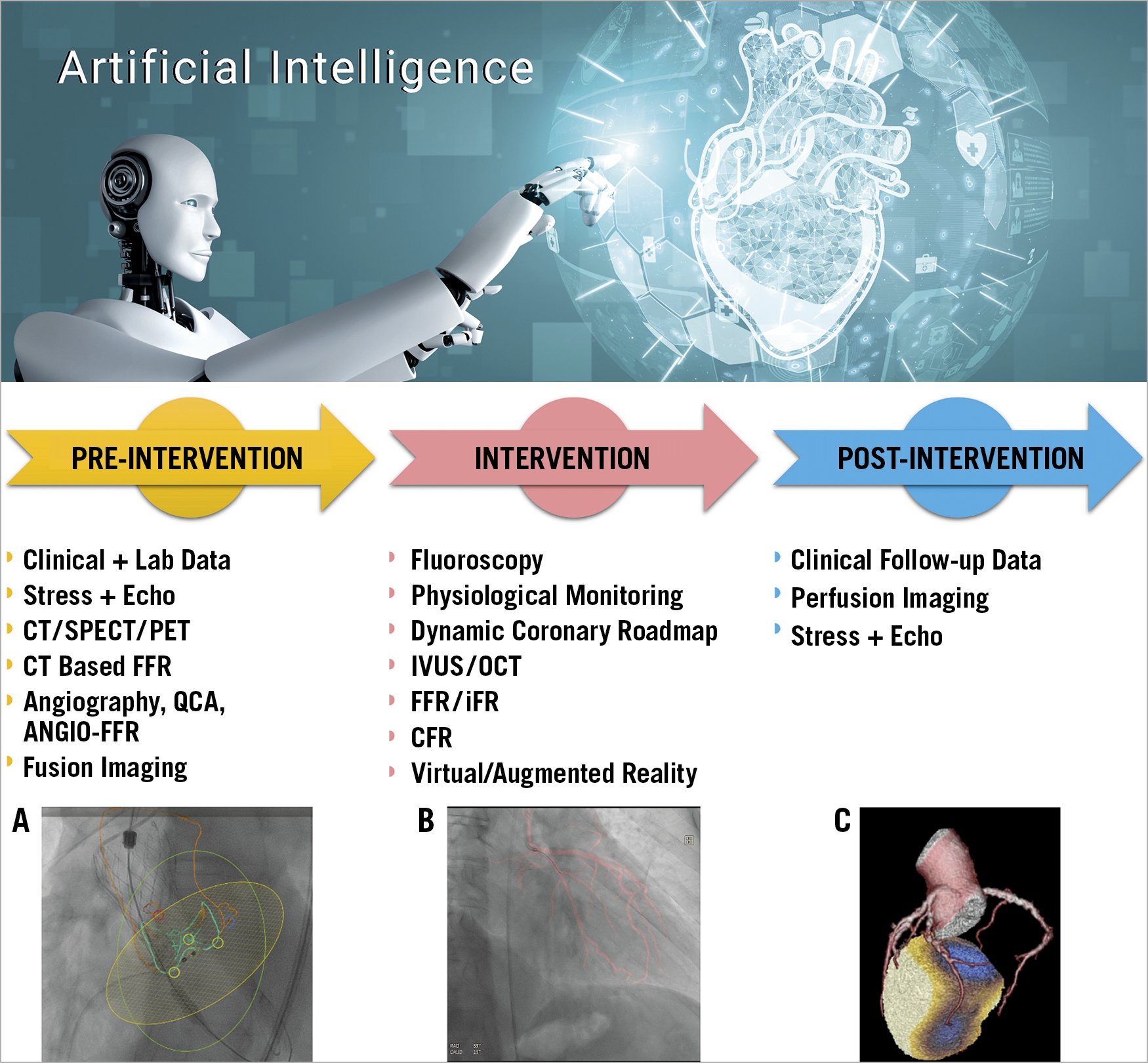
Central illustration. Current imaging and physiology methods pre-, during, and post intervention. Pre-intervention multimodality 3D imaging may be obtained to allow better understanding and planning of the procedure. Fusion of the angiographic images with CT data enhances 3D understanding of procedures such as TAVR (A). During the procedure, fluoroscopy is the main tool for guidance, but various intravascular methods may be combined with angiographic procedures. Dynamic Coronary Roadmap is a novel tool to aid navigation during the procedure (B). Post-procedure imaging varies between different imaging modalities, with or without flow reserve challenges. An example of SPECT CT is shown in panel C. AI can access all these data in the background to aid the physician in planning and execution of an optimal patient intervention.
Digital methods for on-line image analysis and visualisation have been developed to aid the physician during percutaneous coronary interventions (PCI). Since stent visibility is often suboptimal using X-ray fluoroscopy, digital image processing methods for stent enhancement have been developed. A comprehensive review of stent enhancement3 shows its use for assessing stent strut damage, stent overlap, stent failure, aorto-ostial lesions, and bifurcations. Stent enhancement offers ease of use, minimal time required for the analysis, no additional cost, and immediate enhanced image interpretation.
Static vascular roadmaps are regularly used during peripheral interventions where blood vessel movement is minimal. However, in coronary procedures, where motion of the vessels prevents overlay of static roadmaps, navigation within the vessels is done by visual comparison to a displayed angiographic image, and additional contrast injections for pathway verification during wire passage, balloon, and stent placement. The Dynamic Coronary Roadmap (Philips Healthcare, Best, the Netherlands) is a recent development that offers real-time, dynamic overlay of the coronary tree on the fluoroscopic image used for PCI navigation (Central illustration, B). The feasibility of this approach was evaluated in a single-centre study4 which showed that it provides a sufficient roadmap for the majority of patients. Yabe et al5 explored the clinical impact of this technology compared to the traditional approach in a total of 130 consecutive patients undergoing elective PCI. The Dynamic Coronary Roadmap compared to the traditional approach was associated with a significant reduction in contrast volume (22%) and fluoroscopy time (30%), despite similar clinical and procedural characteristics. Clearly, such technologies to assist the physician in better image analysis may have a significant impact on the efficiency of the procedure and on patient outcomes.
Besides standard coronary angiography, the past 20 years have also seen the introduction of other imaging methods to the interventional laboratory, such as intravascular ultrasound (IVUS) and optical coherence tomography (OCT). Outside of the interventional laboratory, cardiovascular imaging advances using multislice three-dimensional (3D) computed tomography (CT) were implemented to advance the planning of complex interventional procedures. A few examples include planning for transcatheter aortic valve replacement6 (Central illustration, A), septal occlusion for treatment of hypertrophic cardiomyopathy, and a clinical decision-making tool that uses flow equations to calculate non-invasive coronary flow reserve7. One of the most challenging image-based coronary artery analyses in the past was the identification of vulnerable plaque8. This challenge led to a number of innovative invasive9,10 and non-invasive11,12 image-based technologies.
Powerful computer technologies have made it possible to merge different imaging modalities13. For example, IVUS and OCT are intrinsically planar, but can be visualised using their original curvature in volumetric 3D as well as dynamically in four dimensions (4D), with the appropriate data set. Besides 3D visualisation, which in itself has major advantages, the reconstructed data can also build patient-specific computer models. These models can be used for additional calculations and analysis using biomechanical flow simulations, which enable shear stress distribution measurements in the coronary arteries. This can be used as a clinical risk predictor for plaque progression or rupture in specific segments8. The addition of AI will help in real-time image analysis, as well as providing real-time clinical, laboratory, and other important information. Figure 1 provides a schematic flow chart of the integration of imaging in the catheterisation laboratory applying AI to support the physician’s decision-making process.
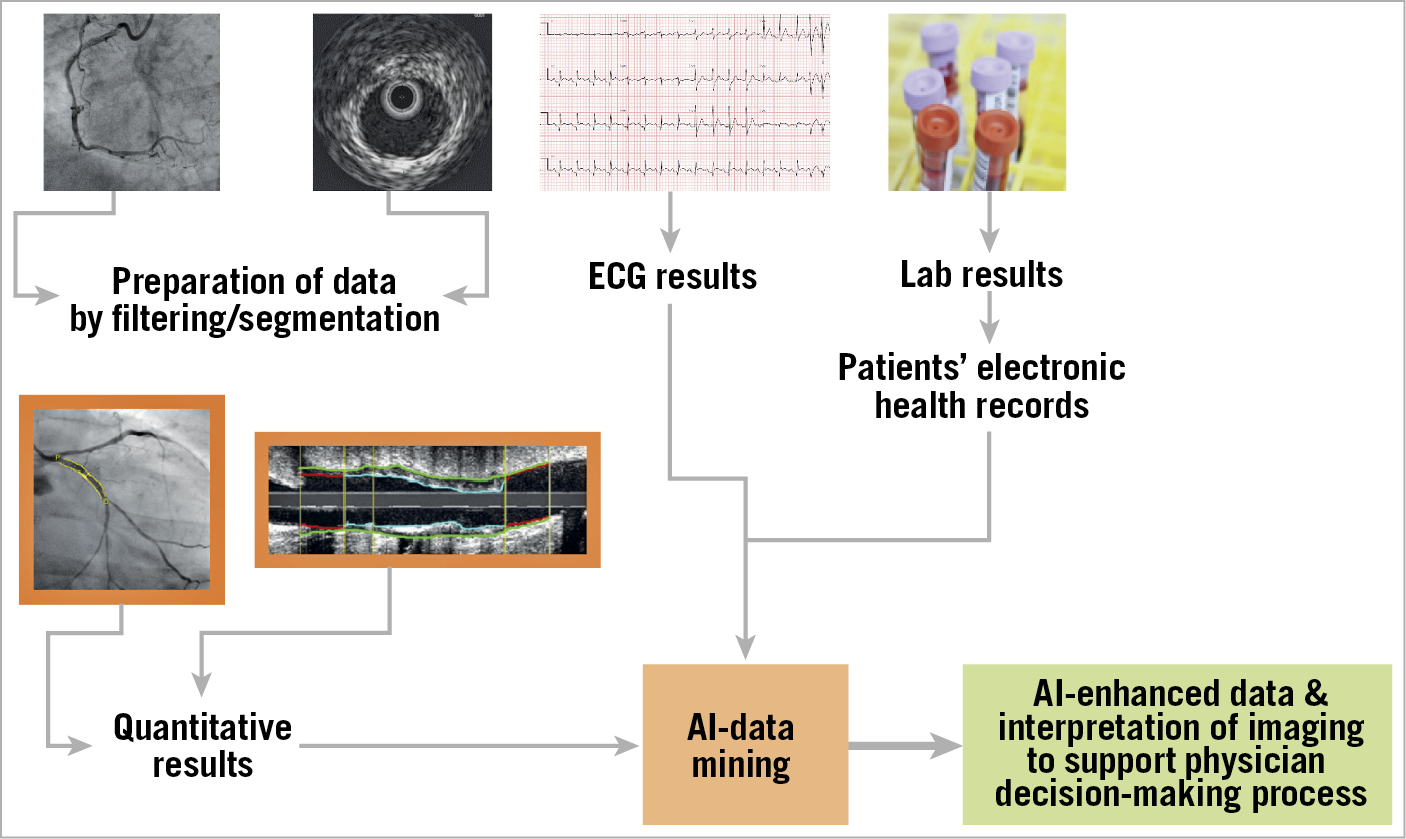
Figure 1. Schematic presentation of the use of artificial intelligence (AI) with data mining in the interventional laboratory. Integration of X-ray and intravascular imaging data, ECG, laboratory results, and the patient’s electronic health records are analysed by AI. Imaging interpretation will be supported by AI and enhanced by real-time clinical, laboratory and other important information to support the physician’s decision-making process.
ARTIFICIAL INTELLIGENCE AND COMPUTATIONAL CARDIOLOGY
The potential benefits of using AI in medicine are now being extensively pursued14. Its implementation and use will dramatically change the medical landscape. There are some promising new and successful applications related to electrocardiogram (ECG) analyses15, as well as some new developments in echocardiography16. Several excellent sources of information regarding the basic principles of AI and its applications in medicine and interventional cardiology are now available17,18.
AI will have utility in analysing the ever-growing amount of patient data being generated. Potentially, leveraging this big data using AI in the catheterisation laboratory will markedly increase prediction accuracy and clinical decision making for several treatment modalities. Currently, available databases are dedicated strictly to the specific imaging modality used, with limited information regarding the clinical and laboratory data of patients. In the future, a critical AI enhancement will be the addition of clinical and laboratory data to the imaging data to enhance system precision further. The algorithms will improve themselves based on machine learning and deep learning algorithms. However, to achieve this goal, large high-quality databases with accurate annotations are needed. Currently, such large data sets of interventional images are generally lacking when, for example, compared to the world of ECG AI analysis19. One proposal for achieving this goal is to build a digital twin of each patient as a computer model that includes all available clinical, imaging, and laboratory data. This, combined with AI analysis, could lead to applications for actualising precision medicine for our patients14.
COMPUTER VISION
While we are already seeing impressive integration of different imaging modalities in the interventional laboratory, they are still limited to predominantly two-dimensional views. Fusion imaging between the various non-invasive imaging modalities, e.g., ultrasound, magnetic resonance imaging (MRI), single-photon emission computed tomography (SPECT), and CT, is difficult to achieve, though significant progress has been made20. An example of a SPECT CT fusion image is shown in the Central illustration, panel C. Fusion of prior CT 3D information with fluoroscopy is of particular benefit in structural heart interventions (Central illustration, A), as well as in certain cases with challenging chronic total occlusions21 that require clear visualisation of the entire vessel.
Fusion of angiographic images with IVUS, OCT, and physiologic measurements has been widely applied. This methodology, which is of interest in research studies that further characterise the atherosclerotic plaque22, has great potential for better guidance and precision in robotic interventions, by superimposing data received from such tools on angiography to achieve full plaque coverage while avoiding unnecessary treatment of non-critical lesions in long diffuse disease.
Commercially available software for registration of both CT and MRI images to fuse rapidly with fluoroscopic imaging is now available (VesselNavigator system; Philips Healthcare)23. This imaging overlay technology enables 3D reconstruction of structures of interest (Central illustration, A), providing guidance for transcatheter interventions in structural procedures. In a single-centre study, intervention for congenital heart diseases with rapid registration proved feasible, aided planning of angiographic angles, and offered precision in guidewire manipulations24.
The next step to more realistic 3D/4D renderings and visualisation could be holography25. However, this requires a prospective reconstruction based on prior CT or MRI before the patient enters the interventional laboratory. The optimal pathway to achieve this would be generation of real-time virtual and mixed reality visualisations. Devices such as the HoloLens™ (Microsoft, Redmond, WA, USA) could provide such a set-up26. Figure 2 provides an example of such a set-up, with the semi-transparent 3D hologram displayed in real time in front of the cardiologist, thereby assisting with the procedure. These ideas have led to a new interdisciplinary field, referred to as “computer vision”, which deals with how computers extract a high level of understanding from digital images, similar to how we as humans do. This technology uses multiple information sources at once, something not possible for the human operator27.
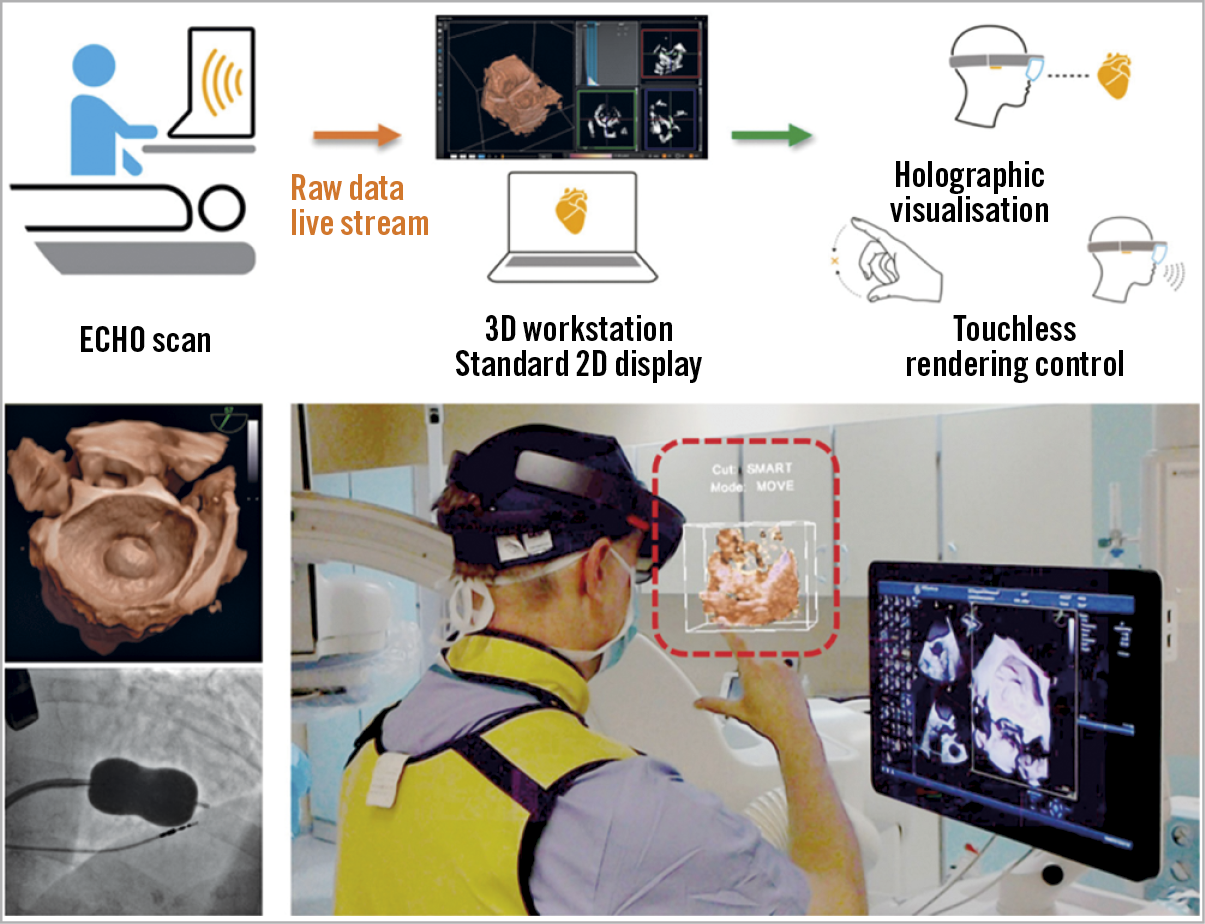
Figure 2. The HoloLens mixed reality display (Microsoft, Redmond, WA, USA) is used to overlay 3D data on a hologram reality view during a balloon mitral valve intervention. Data obtained for ultrasound echocardiography are visible as a semi-transparent holographic cube positioned in front of the echocardiographist and shared by an interventional cardiologist. Reproduced with permission from Kasprazak et al26, and from the European Society of Cardiology. All rights reserved.
ARTIFICIAL INTELLIGENCE AND INTERVENTIONAL IMAGING
Krittanawong et al28 presented an overview of AI in precision cardiovascular medicine. One example, although not widely used today, is that of myocardial blushing in image analysis. However, it may well be possible to use it in the clinical setting, if online AI-based analysis can speed up the analysis process29. Complete reconstruction and analysis of the entire coronary artery tree can be the input for robotically assisted procedures, as described by Sardar et al30. Another important area is the training of interventionists for complex procedures. Cates et al31 presented a computer-based simulation using real patient data as a procedure rehearsal, which could improve actual procedural performance. Several currently practical available deep learning applications for cardiovascular image analysis have been described by Litjens et al32. The number of AI-related developments in interventional cardiology is exploding and exponential growth is expected soon, for example, the use of AI to detect fractional flow reserve through diagnostic angiography automatically33.
MEDICO-LEGAL CONCERNS
Medico-legal issues for AI-based decisions are still under debate. For example, will physicians be legally comfortable with treatment decisions based on AI, since the physician remains liable? The safety and effectiveness of such algorithms have been dealt with recently by the United States Food and Drug Administration (FDA) and European Union (EU), which have issued regulatory guidelines for AI implementations, requiring prior evaluation before AI software can be used as a medical device34. While many questions need to be answered with respect to AI in medicine, two of the main concerns are “black-box” results, which do not necessarily relate to pathophysiological mechanisms, and questions regarding the future role of the physician within the AI environment. We have to ensure that we will not lose our “clinical sense” in getting used to relying on AI-based diagnosis and therapeutics.
FUTURE STEPS
Applying AI technology to big data analysis requires a high degree of standardisation and integration of the various data sources within a hospital, between hospitals, and access to prior data from patient referrals, as well as outcome data from outpatient follow-up. In most countries, each medical institute or group of institutes uses a different patient record system; the integration of imaging databases with clinical and laboratory databases within institutions is limited. Standardisation protocols are required to allow the integration and use of various data sources. This should not be limited to local/domestic sources but extended to include international sources as well.
We must standardise data acquisition and analyses protocols. This in itself is a challenge, since implementation of digital standards within the clinical setting has been slow. By example, the medical imaging standard - Digital Imaging and Communications in Medicine (DICOM) - has been successfully adopted widely35. However, because of the variability of imaging devices, some vendors are not implementing identical protocols of data acquisition in their devices. Using historic data can be problematic, as data “quality” often cannot be controlled. As mentioned earlier, the crucial element here is to create large quality databases with accurate and reliable annotations.
The process of algorithm validation, mainly with application to a specific patient, is complicated and demanding. Medicine in general is a conservative discipline and tends to apply novel methods after thorough corroboration. To ensure that AI can be successfully implemented into our cardiology practice, there must be a commitment to show accuracy through validation processes36,37. Routes to validate the AI-based decision processes should be explored and compared to expert decisions coming out of the catheterisation laboratory. Such validation is clearly a cross-disciplinary effort. Only when this is achieved will physicians have the confidence to embrace AI applications and AI-derived clinical decision-making processes. Finally, disparate components of the medico-legal and ethical considerations need to be resolved38.
FROM X-RAY IMAGING TO RADIATION REDUCTION
In the ongoing effort to provide more advanced transcatheter therapies to our patients, consideration must be given to the potentially detrimental physical effects of cumulative radiation exposure amongst catheter laboratory operators and staff. This is of growing importance, given the increasing complexity of coronary interventional procedures being offered, as well as the continued expansion in structural cardiology procedures in contemporary interventional cardiology practice.
Staff education and training in the fundamental physics of mitigating excess radiation exposure (e.g., maximising the distance from the radiation source, avoiding steep working angulations during procedures, etc.) continues to form the foundation of safe radiation practice in the catheterisation laboratory. However, innovations in technology, particularly at the dawn of the AI era, hold significant promise for optimising fluoroscopic interventional procedures and reducing occupational radiation exposure.
Traditional approaches to radiation reduction, apart from the education of the operators, have primarily involved hardware modifications and shield-based interventions. This attunes all major manufacturers of angiography systems to the need to compete on radiation reduction measures. Accordingly, research and development aimed at improving X-ray tube designs and radiation detector sensitivities is ongoing. Ancillary hardware inventions continue to evolve, often as novel approaches to architectural shielding of the operator from ionising rays. This is being realised by the development of a ceiling-suspended radiation protection system (Zero-Gravity™; Biotronik, Berlin, Germany) that replaces the traditional wearable lead apron with a thick transparent lead glass armour, allowing the operator to reach and manoeuvre the catheters while fully shielded39. Finally, the most radical approach to reducing occupational radiation exposure is the adoption of complete robotic systems that can facilitate the complete removal of the operator from the radiation environment (described below).
Because of the fundamental physical nature of working with ionising radiation, refinements in X-ray hardware technologies will always remain a central target for innovation. However, the catheterisation laboratory of the near future is likely to feature a host of software innovations that will either directly or indirectly reduce radiation exposure. Analogous to the updating of a smartphone’s operating system, software innovations designed to integrate into hardware systems may permit new and extended applications using existing X-ray equipment.
One of the most high-profile examples of recent software innovations for incorporation into the angiography suite is the integration of eye-tracking technology to focus the X-ray beam intuitively towards the region of most interest. The premise of this technology is that it mimics the organisation and function of the human visual system – where the most detailed imaging is required only in the central vision, with the peripheral vision providing less detail. The technology utilises semi-transparent filters to focus the X-ray beam on the area of the image that the operator’s eyes are focused on while delivering less radiation to the peripheral areas where it is not needed. Such an approach in a swine model has reportedly reduced operator irradiation by approximately 75% without interfering with performing fluoroscopically guided interventional procedures40.
Taking computational integration in the catheterisation laboratory to an even higher level, future software innovations will very likely capitalise on AI image processing capabilities. Specifically, AI “computer vision” algorithms can recognise images or features in multidimensional data sets and particularly apply those to fluoroscopic imaging. Early examples of computer vision algorithms have shown promise for real-world use, having been able to show accurate detection of stenosis characteristics, and abnormalities such as dissection or thrombus41. With development, they may then use such labelled data for optimal suggestion of the ideal angiographic projection and to acquire the minimal number of angiographic images to exclude disease, etc., thereby reducing radiation analogous to the practice of an experienced human operator.
Perhaps the ultimate goal of AI in the catheterisation laboratory of the future is the notion of a series of algorithms working together to develop and support human clinical decision making – specifically, the process of computer vision labelled data, being interpreted by machine-learning algorithms to provide intelligent diagnostic and procedural decisions. However, currently, such applications remain in their infancy. One such emerging AI technology relevant to radiation reduction is one of a series of algorithms being developed by Cerebria (St Albans, United Kingdom)42,43. They have trained a neural network to identify automatically the ideal collimation settings of a particular angiographic frame. The potential of such a technology is the automatic and optimal reduction in radiation exposure for patients and staff during procedures, with the additional benefit of improved image quality.
In summary, the catheterisation laboratory of the twenty-first century is likely to provide a significantly lower radiation environment than the current ones. Innovations in hardware, and particularly AI-based software, will ensure the continued provision of transcatheter therapies with increased radiation safety for operators and patients alike.
ROBOTICS AND AUTOMATION
HISTORY OF SURGICAL ROBOTICS
One of the first robotic medical applications was aimed at surgical procedures in the battlefield. Originally developed by DARPA, it aimed to provide a "telepresence" for remote procedures44. Subsequently, a private-public partnership and mergers between companies saw the entry of da Vinci surgical robotics into the medical field, aimed at extending the human interface to improve patient care and outcomes by enabling smaller incisions, improving precision at the surgical site, and enabling remote procedures. The first surgical robotic procedure was performed in 199745 and today robotic surgery is widely used in various surgical and interventional fields. The original concept of remote “telepresence” surgery was first used for a cholecystectomy in 2001 between the USA and France, through a direct transatlantic point-to-point communication line allowing 155 ms lag time46. Interestingly, despite its success, remote surgical procedures did not gain clinical acceptance. Legal and licensing responsibilities, lack of physician to patient interaction, need for specifically dedicated communication lines and the need to resolve unexpected emergencies safely may be some of the reasons why remote surgical robotics has not yet been clinically adopted. Looking to the future, a game changer leading to acceptance may be the stronger unmet need due to the shortage of experts in some medical disciplines, together with technological optimisation of communication speed.
CORONARY ROBOTICS: FROM THE EARLY DAYS TO FDA APPROVAL
The field of PCI was born when Andreas Gruentzig performed the first coronary balloon angioplasty in 197747. Four decades later, the way PCI is performed remains the same, with the operators standing next to the patient within the harsh X-ray environment, partially protected by shields and wearing a lead apron and additional accessories. The hazardous effects of radiation range from a higher likelihood of cancer48, to brain tumours49, cataracts50, and spine problems51, to name but a few.
Coronary robotics evolved to protect the operators and enhance precision. Navigation of coronary wires using a magnetic field52 was tested in patients, with a 93% success rate in 439 lesions53. However, apart from the wire navigation, the rest of the procedure had to proceed manually. The concept of a fully robotic PCI procedure was introduced by Beyar and colleagues54,55, who proposed a remote navigation system, with the wire, balloon, and stent (Figure 3A) manœuvred via a bedside unit using a single joystick on a remote-controlled unit (Figure 3B). After proof of safety and feasibility in animals54, the first cohort of 18 coronary stenosis patients was treated with this system, by robotically navigating the wire across the lesion, advancing the balloon to its position, and performing stent implantation, with post-dilatation as needed. All cases were successful and there was only one complication, i.e., a system malfunction, necessitating a manual procedure instead55. The remote navigation system provided continuous, as well as discrete 1 mm steps of either wire or balloon movements, thereby allowing precise movements of the device for accurate lesion length measurement and stent placement.
![Figure 3. The evolution of the robotic PCI system and concept. A) The original Remote Navigation System manipulating wire and device are controlled at the console by a joystick (B). Reprinted from Beyar et al55, with permission from Elsevier. C) The current CorPath GRX control station60 is positioned within a shielded cockpit in the catheterisation laboratory with the operator console controlling the wire, the device, and the guide catheter (taken during robotic PCI at the Interventional Cardiology Center, Jagiellonian University Hospital, Poland). D) Set-up for the first remote catheterisation performed by Dr Tejas Patel in Ahmadabad, India. The control station is located 35 km away from the catheterisation laboratory, with the robotic arm at the patient side. The video of the patient room and the monitor screen are transmitted via the internet. From Patel et al79 [CC BY-NC-ND 4.0].](https://eurointervention.pcronline.com/storage/issues/EIJ194/097_EIJ-D-21-00145_Beyar_194/medias/222431/03_Beyar_194.jpg)
Figure 3. The evolution of the robotic PCI system and concept. A) The original Remote Navigation System manipulating wire and device are controlled at the console by a joystick (B). Reprinted from Beyar et al55, with permission from Elsevier. C) The current CorPath GRX control station60 is positioned within a shielded cockpit in the catheterisation laboratory with the operator console controlling the wire, the device, and the guide catheter (taken during robotic PCI at the Interventional Cardiology Center, Jagiellonian University Hospital, Poland). D) Set-up for the first remote catheterisation performed by Dr Tejas Patel in Ahmadabad, India. The control station is located 35 km away from the catheterisation laboratory, with the robotic arm at the patient side. The video of the patient room and the monitor screen are transmitted via the internet. From Patel et al79 [CC BY-NC-ND 4.0].
On the basis of that system, the CorPath 200™ system (Corindus Inc., a Siemens Healthineers Company, Waltham, MA, USA) was developed. For this set-up, the bedside unit was hooked to the patient table and the dual control unit (wire and device) was placed in a protected cockpit within the catheterisation laboratory. Following animal experiments, the system was then used in eight patients to prove safety and feasibility56. This was followed by a pivotal study in 164 patients57 that showed 98.8% technical success and 95.2% reduction of radiation to the operator. In 2012, the FDA approved the system based on this study.
BEYOND THE FDA
Extensive use of the robotic system in multiple sites created solid literature regarding the use of the system in real-world scenarios. Experience in complex lesions58 showed that partial or full conversion of the procedure to a manual operation was required in 18.5% of the cases. Since the lack of a guide catheter control was identified as a major limiting factor59, the second-generation system was designed with a triple control (wire, device, and guide catheter) (CorPath® GRX; Corindus Inc.) (Figure 3C) and received FDA approval in 201660. The initial experience in 40 consecutive patients showed a high success rate61. Feasibility of robotic PCI in unprotected left main lesions was reported62, including the use of an Impella® circulatory support system (Abiomed, Danvers, MA, USA) during robotic high-risk angioplasty63. The system also allowed use of phased-array IVUS, laser atherectomy64, and other devices. Comparison of 6- and 12-month outcomes between robotic and manual PCI in complex lesions65 showed equivalence between procedures66. Geographical miss was markedly reduced by robotic assistance67. Positive experience with robotic PCI for chronic total occlusion has also been reported68,69. Recently, the use of robotic PCI in a suspected COVID-19 patient in need of urgent coronary artery interrogation to minimise the risk of viral exposure to the staff was reported70. Application of robotic angioplasty to the femoral-popliteal arteries (RAPID trial) showed 100% success in 20 patients71. Robotic PCI has been reviewed as a paradigm change in interventional cardiology72; however, the need for randomised controlled studies to prove its benefits was clearly indicated73.
Mangels et al74 compared resource utilisation in robotic PCI (n=56) with manual PCI (n=108). They report higher direct supply costs attributed to single-use robotic components, without a significant difference in total hospitalisation or catheterisation laboratory cost for robotic PCI. Similar fluoroscopy time, procedural time, and total number of stents with a lower volume of contrast were reported for robotic PCI.
Recently, fluoroscopy time and contrast use were compared by a propensity score-matched analysis between robotic (310 patients) and manual PCI (686 patients)75. Robotic PCI was associated with a significant reduction in patient exposure to radiation, no increase in fluoroscopy time or in contrast utilisation, and a minor increase in procedure duration compared with traditional PCI.
FROM THE CATH LAB TO THE CONTROL ROOM TO REMOTE INTERNET PROCEDURES
While the majority of interventional procedures are set up with the control station placed within a protected cockpit in the catheterisation laboratory next to the patient table, some operators prefer performing PCI with the console placed in the control room. The concept of a remote procedure, during which the operator is outside and potentially distant from the patient procedure room was initially tested in a swine model within the Mayo Clinic, using local communication76 with full success in 52 experiments. Latency effects for up to 1,000 ms were tested; a detrimental effect was observed only for a latency greater than 250 ms. In parallel, Madder and colleagues77 showed the feasibility of remote telestenting through a hard-wired connection to an adjacent room in 20 patients, with 95% success. Following prior simulation and in vivo evaluation of robotic PCI over the internet78, the first remote cases were performed by Dr Patel in Ahmadabad, India, who performed PCI in five patients from 35 km away through a regular wired connection79. The remote operator worked at the standard control station and was able to see the entire catheterisation laboratory, the angiographic images, and the haemodynamic monitors. The set-up of the remote site with Dr Patel is shown in Figure 3D. Internet connection allowed streaming video visualisation of the procedure and uneventful communications between the remote site and the catheterisation laboratory. A diagram of modes of remote operation is shown in Figure 4. In the catheterisation laboratory, the robotic arm is hard-wired to the operator console where it can be placed in a shielded cockpit next to the patient or in the adjacent control room. In addition, a duplicate console in a remote location can be operated by experienced interventionalists through standard internet, 5G, or other fast and broadband communication channels.
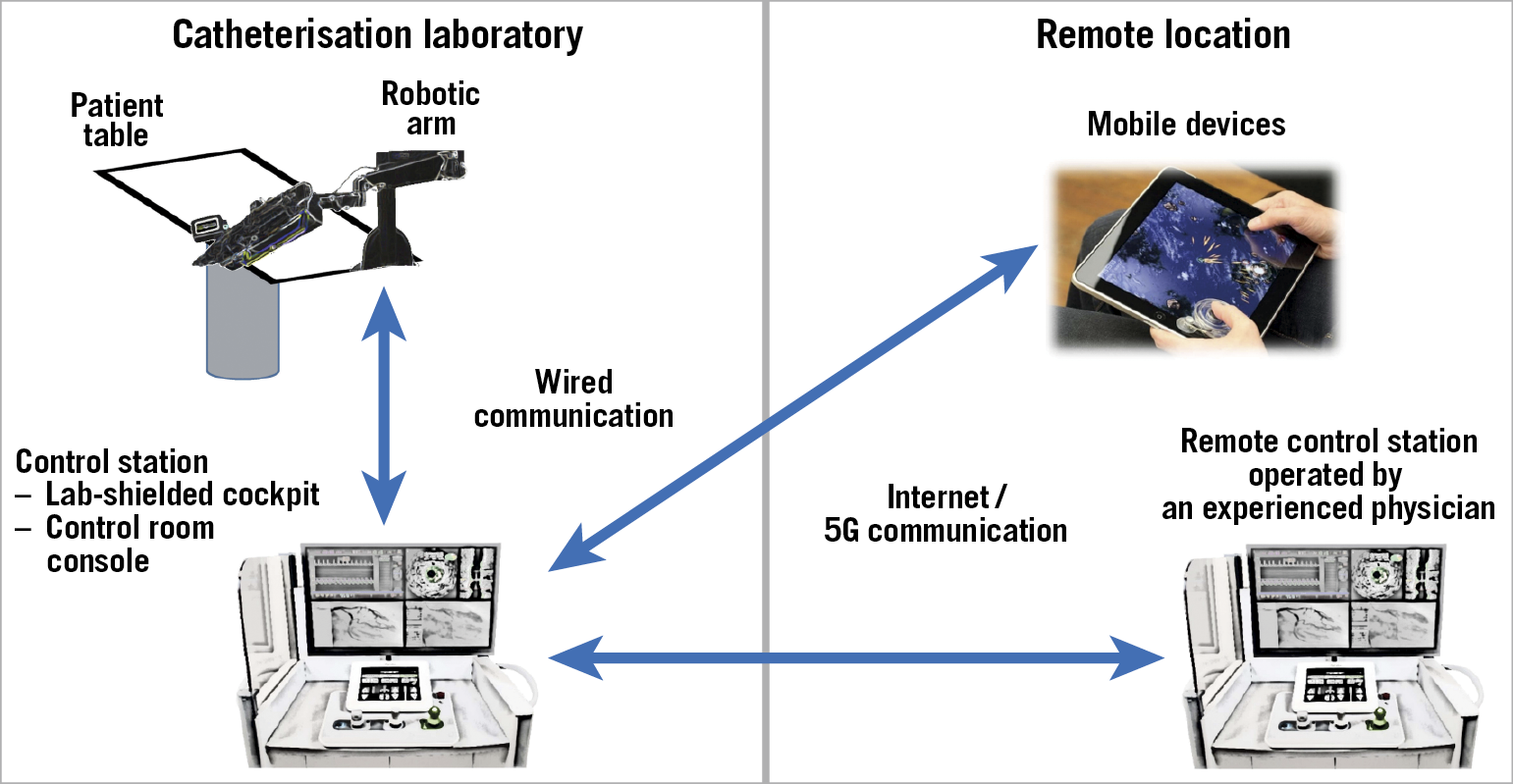
Figure 4. Local and remote schematics. The robotic arm, located in the catheterisation laboratory, is hard-wired to the control station, which can be placed either in the catheterisation laboratory or in the control room, according to the operator’s preference. A second remote control unit, which can also be installed on a mobile device, is connected through the internet or a 5G wireless connection and can be placed in any other location (i.e., either elsewhere in the same or a different hospital, or any location worldwide).
The ability to perform efficient and safe procedures over the internet depends on the lag time, which is unfelt for latencies less than 250 ms. The measured latency in the human study was 53 ms80. Recently, 5G wireless networks have been opening a whole range of possibilities for remote procedures. A test performed across the USA using the 5G network indicated the feasibility of performing robotic procedures remotely with a measured latency of 121 ms compared to 67.8 ms in a wired connection, both imperceptible latencies80. The remote experience in surgery and PCI clarifies that the technical aspects for remote PCI have been solved for both wired and wireless communication. Other aspects of remote operation, including licensing, liabilities, and the required presence and level of expertise of the staff at the remote site require further investigation. User experience of remote interventions includes a combination of speech and image synchronisation. Currently, latency can be a problem when transferring over long distances. Future developments must overcome this issue and facilitate an immediate response of the robotic system with undetectable latency. Further evaluation in practical clinical settings over long distances is required if we are to see an increase in the implementation of remote procedures. Obviously, the ability to manage problems such as system failure or broken communication safely should be a mandatory requirement.
ROBOTIC NEUROINTERVENTIONS
Robotics for vascular neurointerventions based on the cardiovascular experience have been discussed previously81. The CorPath GRX system has already been successfully applied to the neurovascular area by advancing the microcatheter, micro stent, and multiple coils to close a large aneurysm in the basilar artery82. The researchers concluded that neuro-endovascular intervention using robotic assistance is feasible, offers improved precision, and opens up future possibilities for remote interventions, such as emergency thrombectomy for stroke.
While the CorPath GRX can accommodate most available devices, a dedicated neurovascular system is needed that can handle the major devices used in this field and provide remote internet capabilities. Madder et al80 have pointed out that such a system could be used to solve the problem of geographical disparities and the lack of expert neurovascular interventionalists, as previously discussed83. Rabinovich et al84 discussed current and future directions using robotics for neurointerventions. AI-enhanced robotic neurointerventions have the capability to uncover new dimensions within the realm of cerebrovascular therapeutics.
AUTOMATION
Automated wire manipulation can be a major tool to enhance procedure efficiency by the operators. A special “retract and rotate” feature has already been included in the current CorPath GRX system, and is good for navigating through side branches85. Other wire navigation features such as spin and wiggle, as well as device “dottering” applications have already been implemented and used in patients86. Visualisation and navigation using virtual reality could be combined with robotics87, and full automation of wire navigation via image-based analysis and machine learning would enhance our performance and provide important platforms for parts of the procedure30. The next clinical trials should be planned, not only to prove the non-inferiority of robotic PCI, but also to determine definitively the superiority of robotic versus manual PCI, as well as to define possible measurable clinical endpoints to demonstrate overall benefit for both patients and medical staff.
We anticipate a number of benefits from using AI for robotic PCI: software training of new and intermediate users for optimal device manipulation based on the tracked movements of highly skilled operators, advanced integration with the tracking of other robotic coronary devices, development of on-line computational methods for precise selection of projection angles, and solutions for decreasing X-ray dose as well as procedure time.
HAPTICS
Compensating for the lack of haptics in today's endovascular interventions is the precise and detailed visual feedback during fluoroscopy of the wire tip, stent, and guiding catheter movements. Nevertheless, there may be a need for haptic feedback in certain procedures such as total occlusions, where the pressure of a stiff wire is clearly felt by the operator. The safety of wire manipulation using the robotic arm with software programmed movements needs to be fully examined, both with and without haptics, to determine adverse outcomes such as dissections or perforation.
THE FUTURE OF VASCULAR ROBOTICS
Overviewing the milestones achieved in robotic PCI since the original concept of remote control navigation for coronary interventions54,55 shows that the original coronary applications, which were improved following the FDA-approved versions57,60, have further expanded to peripheral, carotid, and intracranial procedures. The remote milestone achieved in India79 needs to be further assessed in well-designed controlled studies in order to show its added value to expand the access to urgent stroke care. It is clear that robotic coronary, endovascular, and neuro-endovascular interventions are going to have increasing roles in the catheterisation laboratories of the future88.
There are already additional endovascular robotic technologies that are in various phases of development. The R-One™ system (Robocath, Rouen, France) is in clinical trials in Europe, and the Liberty™ disposable vascular robot (Microbot, Yokneam, Israel) is in the preclinical phase of development. Patient benefit, radiation protection, and ergonomics will be the leading factors for future robotics.
The ability to perform remote procedures has been proven for both wired and wireless communication. Enhanced by partial automation using machine learning and AI, remote operations may become an integral part of our interventional procedures. We anticipate a number of benefits to the operators and eventually to our patients. One such benefit is the possibility to standardise performance of PCI by using advanced robotic controls to guide and move coronary wires in complex cases and for side branch access, with less dependence on operator experience. In addition, AI and machine learning provide opportunities for better outcomes by enabling good preprocedural PCI planning. In addition, it may allow remote guidance for the local operator by an experienced distant operator, or even performing of PCI by a skilled operator via full remote guidance. Furthermore, remote procedures may allow the provision of advanced emergency coronary or neurological interventions by enabling access to top operators in different time zones, as well as offering advanced coronary, peripheral, and neurological interventions by skilled operators in underserved areas with a limited number of highly specialised doctors.
The potential for robotic percutaneous valvular interventions has not yet been applied to patients. Surgical robotic techniques for mitral valve repair and coronary bypass surgery have been studied previously but are not widely used except in highly dedicated centres. Robotics will also play an important role in the future education and training of students, fellows of coronary, peripheral, and neurointerventional procedures, as well as the nurses and technicians who are involved in robotic interventions in catheterisation laboratories.
SUMMARY AND CONCLUSION
The basics of transcatheter interventions rest in our ability to visualise, diagnose, and interpret the X-ray images obtained throughout the procedure. This review, focusing on coronary interventions, has presented a continuum of progress in the cardiac catheterisation laboratory, aimed at improving patient care in interventional cardiology. Image acquisition and presentation in the catheterisation laboratory is constantly improving, and there has been major progress in reducing the amount of radiation exposure to both staff and patients. Co-registration of multimodality imaging may have an increasing role in the future. AI and machine learning are being used today in the research and clinical settings, and both have great potential for becoming tools central to our decision-making process. Robotics has finally entered the realm of coronary interventions and is becoming an important tool in the catheterisation laboratory, protecting operators from radiation and facilitating precise complex procedures without compromising safety. The potential for AI-enhanced operations and remote robotics across the internet might add to our future practice in remote areas, for cardiovascular and coronary interventions, as well as in neural interventions for stroke, aneurysm, and more.
An overview of such an integrated approach was presented by Sardar et al30 and is shown in Figure 5. Our ultimate goal is to achieve the integration of multiple imaging modalities, AI, online clinical decision support systems, voice-powered virtual assistants, augmented reality platforms, and semi-autonomous/autonomous robotic systems that will become the backbone of individualised patient care.
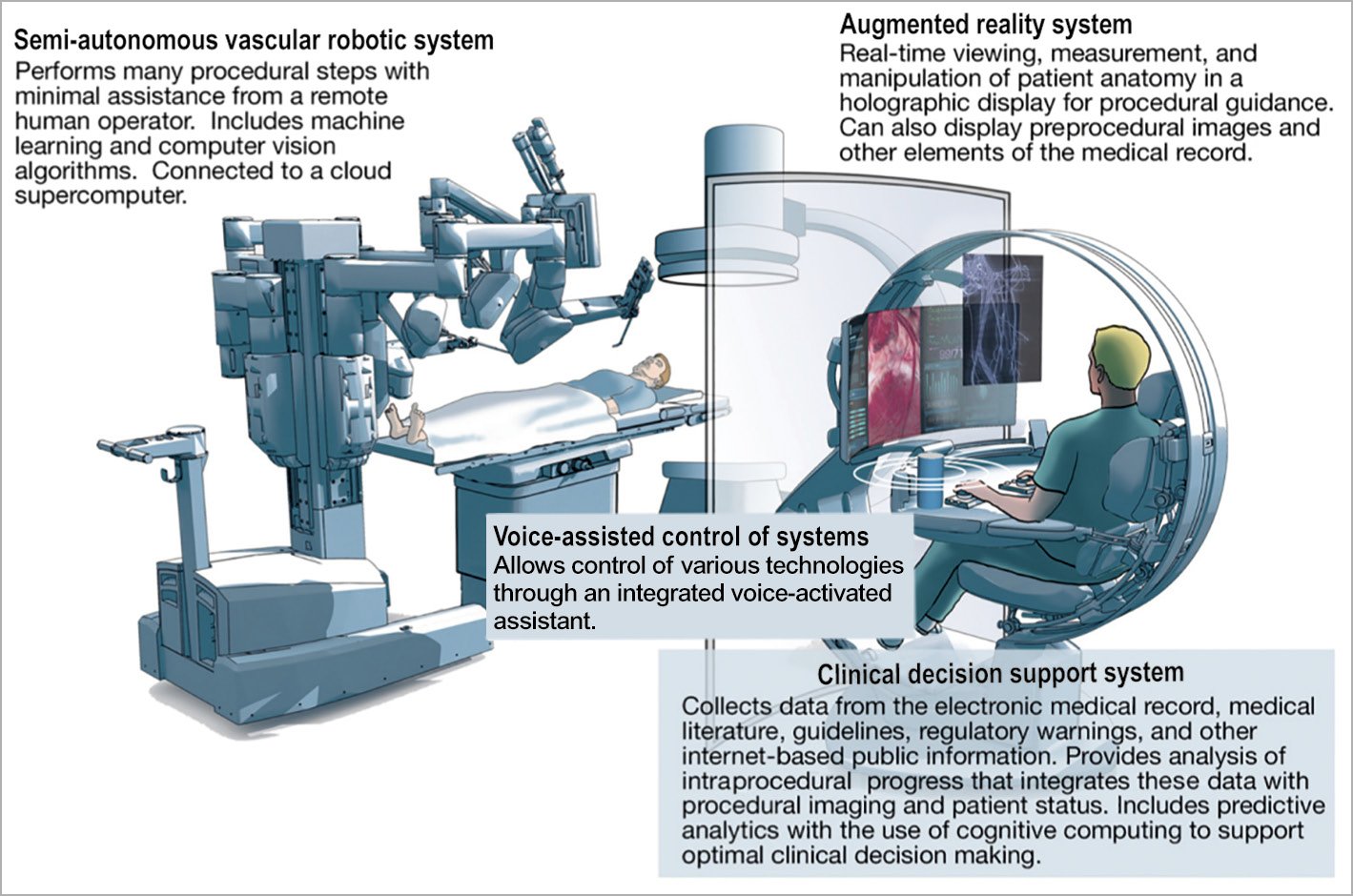
Figure 5. The future catheterisation laboratory with artificial intelligence-enabled technology, clinical decision support system, voice-powered virtual assistant, and augmented reality platforms. A semi-autonomous/autonomous robotic system can provide optimisation as well as the remote operations presented above. Reprinted from Figure 2 of Sardar et al30, copyright (2019), with permission from Elsevier.
In conclusion, advances in imaging in the catheterisation laboratory are revolutionising the way we process and interpret angiographic X-ray images, integrate them with other imaging modalities, and use AI for better diagnosis and management. In parallel, novel methods for image processing and intelligent systems are being used to reduce X-ray exposure. Robotics has begun to enter the field of interventional cardiology and is now expanding towards peripheral vessels and neurovascular interventions, allowing dramatic radiation reduction, optimal and convenient visibility with subsequent enhanced precision, and preparing the catheterisation laboratory for automation using AI and machine learning.
Conflict of interest statement
R. Beyar is the co-founder of Corindus, though he has no relationship with the company today; he also holds equity in and is the director/stockholder at MedHub, CardiacSense, Codiguide, Magenta, Cardiac Success, and Bio-T. C. Cook is a consultant for Philips and Boston Scientific, and has equity in Cerebria. D. Dudek has received grants and personal fees from the following entities: Boston, Philips, Abbott, Medtronic, and Biotronik. He has received grants only from Terumo and Bracco. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

