Abstract
Background: R-One is a robotic percutaneous coronary intervention (PCI) system (CE mark 2019) designed to reduce operator radiation exposure, improve ergonomics, and accurately navigate, position, and deliver guidewires/devices during PCI.
Aims: We aimed to evaluate the safety and efficacy of the R-One system for PCI.
Methods: The European multicentre prospective R-EVOLUTION study included patients with a de novo coronary artery stenosis (length <38 mm, reference diameter 2.5-4.0 mm) undergoing stent implantation. Patients with recent ST-segment elevation myocardial infarction, ostial or left main lesion, bifurcation, severe tortuosity, or calcification were excluded. Clinical success was defined as the absence of major intraprocedural complications. Technical success was defined as the successful advancement and retraction of all PCI devices (guidewires/balloon/stents) without total manual conversion. Radiation exposure to patients, to a simulated manual operator, and to robotic PCI operators was measured.
Results: Sixty-two consecutive patients (B2/C lesions: 25.0% [16/64]) underwent robotic PCI. Radial access was used in 96.8% (60/62) of procedures. The mean robotic procedure duration was 19.9±9.6 min and the mean fluoroscopy time was 10.3±5.4 min. Clinical success was 100% with no complications at 30 days. Technical success was 95.2% (59/62). Total manual conversion was required in 4.8% (3/62) cases, with 1 case directly related to the robotic system. Operator radiation exposure was reduced by 84.5% under and 77.1% on top of the lead apron, compared to doses received on the patient table.
Conclusions: This study suggests that robotic PCI using R-One is safe and effective with markedly lower radiation exposure to the operator. Further studies are needed to evaluate R-One in larger patient populations with more complex lesions. (ClinicalTrials.gov: NCT04163393)
Introduction
Coronary angioplasty for patients with coronary artery disease (CAD) has undergone many procedural improvements since its introduction1. However, the current practice of percutaneous coronary intervention (PCI) remains largely unchanged for interventionalists, who work in a standing position wearing heavy lead protective garments viewing fluoroscopic images from across the procedure table. Repeated exposure to fluoroscopic radiation puts interventionalists and staff at risk, with well-known health consequences including DNA damage and cancer2345678910. Orthopaedic complications from long-term use of heavy lead aprons are also common11121314, resulting in lost workdays and decreased performance1516. The demand for interventionalists is projected to increase with an ageing population and an increase in patients with CAD17.
Robotic PCI addresses these challenges by significantly reducing radiation exposure to the operator1819 and improving ergonomics. Additionally, the fluoroscopic monitors are placed in closer proximity to the operator, providing detailed visual feedback during the procedure. Robotic PCI is also designed to allow more accurate navigation through tortuous vessel anatomy with millimetre precision in manoeuvring wires and devices, with the potential to improve procedural and clinical outcomes for patients.
The feasibility, safety, and efficacy of robotic PCI have been shown in studies assessing the CorPath 200 and the CorPath GRX (Corindus/Siemens)202122. Clinical studies using these robotic systems have demonstrated results comparable to manual PCI23, even in complex coronary lesions, while providing the above-mentioned advantages to interventional cardiologists2122.
In 2019, the R-One robotic system (Robocath) for PCI received CE mark (European conformity) approval, with the first patient procedure performed in France in September 2019. Here, we report the results of the R-EVOLUTION (R-One Efficiency for PCI Evolution With Robotic Assistance) study, the first multicentre study conducted in Europe evaluating the safety and efficacy of the novel R-One robotic system for PCI in de novo coronary artery stenosis patients undergoing stent placement.
Methods
Study design and patient population
This prospective, multicentre, single-arm clinical study was conducted at 6 cardiology centres in 4 countries: France, Belgium, Luxembourg and the Netherlands, from September 2019 to November 2021. A total of 66 consecutive patients who met the inclusion criteria were initially enrolled. Inclusion and exclusion criteria are listed in Table 1. Patients with complex CAD were considered for the study, but complex lesions were successfully treated in a different procedure prior to undergoing robotic PCI of the target lesion(s).
All patients signed an informed consent form prior to inclusion. Patients were included in the intention-to-treat (ITT) population if all eligibility criteria were met, the lesion was deemed treatable, and the guiding catheter was in place. The study duration was 31 months (extended from the anticipated 17 months due to the COVID-19 pandemic).
The study was approved by the respective ethics committees of the involved countries/hospitals.
Table 1. Patient inclusion and exclusion criteria.
| Inclusion criteria: | |
|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Exclusion criteria: | |
|
|
|
|
|
|
|
1) a bifurcation requiring balloon or stent implantation of the side branch (RVD ≥1.5 mm with stenosis ≥50% at or within 5 mm from its origin, or RVD ≥2.0 mm regardless of the presence of side branch disease) |
| 2) located in left main coronary artery, or any left main stenosis >30% | |
| 3) within 5 mm of the ostial left anterior descending artery (LAD), ostial left circumflex artery (LCx) or ostial right coronary artery (RCA) | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Description of device
The R-One robotic system is a fully integrated robotic platform for the remote and accurate navigation, positioning, and delivery of guidewires, balloons, and stents during PCI. An overall schematic of the device is provided in Figure 1. The system comprises a radio-protected control station and a telemanipulated robotic unit mounted with a single-use sterile cassette. Devices are loaded into the robotic unit, with 1 track dedicated to the guidewire and 1 track for the stent/balloon. Motorised modules provide translational and rotational movement to the devices. A standby path is also available for a potential additional guidewire and/or stent/balloon catheter. The interventional cardiologist manipulates the device with joysticks (1 for the guidewire and 1 for the stent/balloon) while sitting at the radio-protected control station located away from the radiation source in the catheterisation laboratory. Fluoroscopy command, haemodynamics, patient table and C-arm commands, and live and reference image duplications are provided at the radioprotection control station. The R-One system is compatible with all commercially available 0.014” guidewires and rapid exchange stent/balloon catheters.
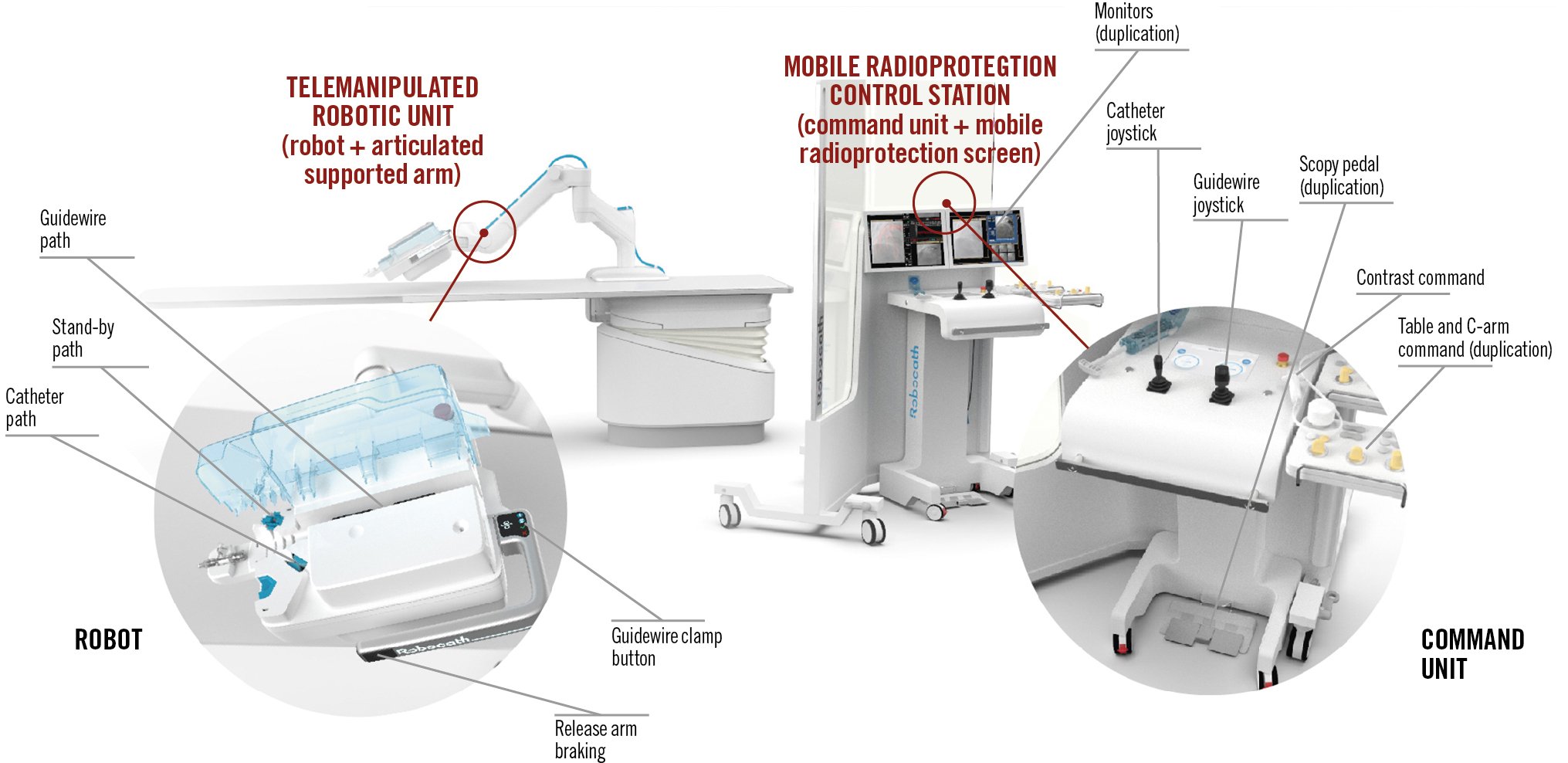
Figure 1. Schematic of the R-One system. The R-One system includes the telemanipulated robotic unit and the mobile radioprotection control station. The telemanipulated robotic unit includes the robot and the articulated supported arm. The single-use, sterile cassette includes a catheter path, standby path, guidewire path, guidewire clamp button, and release arm brake. The mobile radioprotection control station includes a command unit and the mobile radioprotection screen. The control station houses the monitors, the catheter joystick, the guidewire joystick, a scopy pedal, contrast command, and table and C-arm command.
Interventional procedure
Robotic-PCI operators were trained in PCI device implantation using the R-One. Early experience centres (3/6) were defined as having performed 5 robotic-PCI procedures with the R-One prior to patient enrolment for this study, while experienced centres (3/6) had performed more than 5 PCI procedures with the R-One. The choice of device(s) (stent, balloon, guidewire) was made per current practice guidelines, and the R-One was used according to the manufacturer’s instructions.
The procedure starts with standard manual techniques by first obtaining vascular access, then introducing and positioning the guide catheter at the ostium of the targeted coronary artery. The Y connector is then fixed to the cassette and the guidewire is inserted into the robotic unit, beginning the robotic portion of the procedure. The system allows the operator to switch easily and quickly to manual operation if needed.
Study outcomes
The primary safety outcome was clinical success, defined as the absence of intraprocedural complications, including coronary dissection ≥type D according to the National Heart, Lung, and Blood Institute (NHLBI) classification, coronary perforation, decreased Thrombolysis In Myocardial Infarction (TIMI) flow to ≤2, acute vessel occlusion, visible thrombus formation, significant air embolus during the procedure, and traumatic aortic or left main dissection by the guiding catheter.
The primary efficacy outcome was procedural technical success, defined as the successful advancement and retraction of all PCI devices (guidewires, balloon catheters, and stents) and the successful treatment of all the target lesions using the R-One system without total conversion to manual operation. Partial manual assistance was defined as temporary manual operation at the robotic platform and not using the robot to manipulate PCI devices. Total manual conversion was defined as the inability to advance, retract, or rotate devices with the robotic unit, or any other situation where manual conversion was required (e.g., required device was not compatible with the robot, clinical complication, etc.).
Secondary outcomes included the procedure duration, defined as the time between introducer sheath placement and removal; the robotic procedure duration, defined as the time between the first robotic manipulation of the guidewire and the last guidewire removal; contrast volume; bleeding or vascular complications at hospital discharge; and device-related composite criteria (Academic Research Consortium-2)24, defined as cardiovascular death, myocardial infarction (periprocedural and spontaneous) not clearly attributed to a non-target or clinically driven target lesion, or revascularisation at hospital discharge and at 30-day follow-up.
Patients were followed up after hospital discharge to 30 days (±7 days) after the index procedure by telephone to determine anginal status and adverse events.
Radiation exposure subanalyses
Radiation exposure to the patient was obtained from the C-arm.
A simulated manual operator and the robotic-PCI operator were measured as shown in Supplementary Figure 1. Radiation measurements were monitored by Dosilab (Villeurbanne, France).
To measure radiation exposure to the simulated manual operator, dosimeters A and B were located on a pole 1-2 metres from the patient table; they measured radiation exposure on top of a lead apron (A) and underneath a lead apron (B); a piece of lead apron was positioned in front of dosimeter B for the duration of the procedure. Dosimeter readings were multiplied by 4 or 16 depending on the distance from the patient.
To measure radiation exposure to the robotic-PCI operator, dosimeters E and F were used.
Dosimeters E and F measured radiation exposure for the operator during the overall procedure; a piece of lead apron was positioned in front of dosimeter F. Dosimeters E and F were worn by the operator on top of and underneath their lead apron, respectively, while sitting at the control station.
Operator radiation dose ON TOP of the lead apron received during the overall procedure=E.
Operator radiation dose UNDER the lead apron received during the overall procedure=F.
Note: in 2 centres, the operators remained in non-sterile conditions behind the cockpit while a fellow performed the guiding catheter insertion manually. To calculate the total operator radiation dose, the dose received on the simulated operator during the guide catheter insertion was added to the dose on top of/under the operator’s apron during the procedure.
To calculate the dose received during the guide catheter insertion, dosimeters C and D were used.
Dosimeters C and D measured radiation exposure for the operator only once the robotic steps of the procedure had been started, which means they were activated only once the guiding catheter was inserted and positioned; a piece of lead apron was positioned in front of dosimeter D. Dosimeter readings from C and D were multiplied by 4 or 16 (depending on the distance from the patient).
Thus, the operator radiation dose received during the procedure was calculated as follows: the sum of:
- the operator dose during the guiding catheter insertion and positioning (A−C) or (B−D) and,
- the operator dose received during the robot use until the device removal (E) or (F)
This means:
Operator radiation dose ON TOP of the lead apron received during the overall procedure=(A−C)+E
Operator radiation dose UNDER the lead apron received during the overall procedure=(B−D)+F
Statistical analysis
The ITT dataset was used for analysis. Descriptive statistics were calculated and are presented as counts and incidence rates for categorical variables, and as mean, standard deviation, and number of observations for continuous variables. Statistical significance was accepted when p<0.05. Statistical analysis was performed using SAS software, version 9.4 (SAS).
Results
A total of 66 consecutive patients were enrolled in the study, but 4 were excluded because of unmet inclusion criteria (severe tortuosity [n=2], lesion length >38 mm [n=1], >1 lesion per vessel [n=1]). Final enrolment included 62 patients with 64 lesions from 6 sites who met the inclusion criteria and underwent robotic PCI.
Baseline clinical characteristics of the study population are detailed in Table 2. The mean age was 65.4±10.1 years, and 80.6% (50/62) were male. Fifteen (24.2%) patients had a history of PCI. The majority of patients (49/62, 79.0%) presented with a chronic coronary syndrome, such as silent ischaemia or stable angina, and 21.0% (13/62) had an acute coronary event, such as unstable angina or non-ST-segment elevation myocardial infarction (NSTEMI).
Angiographic and procedural characteristics are detailed in Table 3. A radial approach was used in 96.8% (60/62) of cases, all using 6 Fr guiding catheters. The distribution of lesions among the 3 coronary arteries was 34.4% (22/64) in the left anterior descending artery, 32.8% (21/64) in the left circumflex coronary artery, and 26.5% (17/64) in the right coronary artery; 25% (16/64) of the lesions were classified as B2 or C according to the American College of Cardiology/American Heart Association classification. Predilatation was performed in 38/64 treated lesions (59.4%) and post-dilatation in 24/64 (37.5%). Drug-eluting stents were used in all cases, and 96.7% (60/62) of cases used only 1 stent. The mean duration of the robotic procedure was 19.9±9.6 minutes. The mean fluoroscopy time was 10.3±5.3 minutes, and the mean total contrast volume was 118.2±47.3 millilitres.
Primary safety and efficacy endpoints are detailed in Table 4 and the Central illustration. A clinical success rate of 100% was achieved with no major intraprocedural or 30-day complications. A technical success rate of 95.2% (59/62) was achieved. Total manual conversion was required in 3/62 cases (4.8%), and only one was directly related to the robotic system. In this case, the robotic system successfully crossed the lesion with the guidewire but was unable to cross the lesion with the balloon, which was related to a lack of guiding catheter support. Total manual conversion required the use of a guiding catheter extension (GuideLiner; Teleflex), and the procedure was completed successfully with predilatation and stent implantation. The 2 remaining total manual conversions were not due to robotic failure. In the first case, after successful advancement of the guidewire and balloon predilatation, an incorrect manual adjustment of the guidewire into the pads of the robot led to an error detection and temporary system unavailability, resulting in manual conversion. In the second case, the entire procedure was successfully completed robotically, but the angiographic control revealed a non-occlusive coronary dissection (NHLBI type B) not related to the robot. The operator converted to manual and successfully treated the coronary dissection with a second stent. An analysis by centre experience level (early vs experienced) is presented in Supplementary Table 1, and all total manual conversions occurred in early experienced centres. Additionally, the duration of the robotic procedure was shorter in experienced centres (17.47±8.02 minutes) compared to those without robotic-PCI experience prior to this study (22.23±10.99 minutes; p=0.07) (Supplementary Table 1).
Patient and operator radiation exposure data are detailed in Table 5. Patient radiation exposure was 540.3±498.4 milligrays (mGy). The simulated manual operator radiation exposure during the overall procedure on top of the lead apron was 57.1±61.2 microsieverts (μSv) and under the lead apron was 3.2±4.1 μSv. The total calculated robotic operator radiation exposure on top of the lead apron was 7.2±8.7 μSv and under the lead apron was 0.2±0.6 μSv. Robotic PCI operators experienced a reduction of radiation exposure of 77.1% on top of the lead apron and 84.5% under the lead apron compared to the simulated manual operator. For centres who had a secondary operator at the patient table (n=2), the mean operator dose was 0 μSv.
Table 2. Baseline clinical characteristics.
| Variables | Overall population (N=62) |
|
|---|---|---|
| Age, years | 65.4±10.1 | |
| Male | 50 (80.6) | |
| BMI, kg/m² | 27.2±4.7 | |
| Risk factors | Current smoker | 14 (22.6) |
| Diabetes | 17 (27.4) | |
| Hypercholesterolaemia | 35 (56.4) | |
| Hypertension | 33 (53.2) | |
| Family history of CAD | 19 (30.7) | |
| Medical history | 9 (14.5) | |
| Previous myocardial infarction | 15 (24.2) | |
| Previous percutaneous coronary intervention | 2 (3.2) | |
| Previous CABG | 5 (8.1) | |
| History of cerebrovascular disease | 5 (8.1) | |
| Peripheral artery disease | 8 (12.9) | |
| Chronic renal failure | 4 (6.5) | |
| Clinical presentation | Silent ischaemia | 23 (37.1) |
| Stable angina | 26 (41.9) | |
| Unstable angina | 6 (9.7) | |
| NSTEMI | 7 (11.3) | |
| Data are mean±SD or n (%). BMI: body mass index; CABG: coronary artery bypass surgery; CAD: coronary artery disease; NSTEMI: non-ST-segment elevation myocardial infarction; SD: standard deviation | ||
Table 3. Angiographic and procedural characteristics.
| Variables | Overall population | |
|---|---|---|
| Approach (N=62 patients) | Right radial artery | 50 (80.6) |
| Left radial artery | 10 (16.1) | |
| Right femoral artery | 2 (3.2) | |
| Left femoral artery | 0 (0) | |
| Lesion location (N=64 lesions) | LAD | 22 (34.4) |
| LCx | 21 (32.8) | |
| RCA | 17 (26.5) | |
| Other (Ramus) | 4 (6.3) | |
| Lesion class (ACC/AHA) (N=64 lesions) | A | 11 (17.2) |
| B1 | 37 (57.8) | |
| B2 | 13 (20.3) | |
| C | 3 (4.7) | |
| Percutaneous coronary intervention (N=64 lesions) | Sheath size (6 Fr) (N=62 patients) | 62 (100) |
| Predilatation | 38 (59.4) | |
| Stent per lesion, n | 1.05±0.28 | |
| Drug-eluting stent | 67 (100) | |
| Stent diameter, mm | 3.0±0.4 | |
| Stent length, mm | 19.5±6.5 | |
| Post-dilatation | 24 (37.5) | |
| Duration, minutes (N=62 patients) | Total | 39.9±14.6 |
| Robotic | 19.9±9.6 | |
| Fluoroscopy time, minutes (N=62 patients) | 10.3±5.3 | |
| Contrast volume, mL (N=62 patients) | Total | 118.2±47.3 |
| Robotic | 87.4±35.5 | |
| Medications (N=62 patients) | Aspirin | 56 (90.3) |
| Clopidogrel | 49 (79.0) | |
| Ticagrelor | 8 (12.9) | |
| Prasugrel | 5 (8.1) | |
|
Data are mean±SD or n (%). ACC/AHA: American College of Cardiology/American Heart Association; LAD: left anterior descending artery; LCx: left circumflex artery; RCA: right coronary artery
|
||
Table 4. Safety and efficacy endpoints.
| Variables | Overall population (N=62 patients) |
|
|---|---|---|
| 30-day safety endpoint | 0 (0) | |
| Coronary dissection >NHLBI type D | 0 (0) | |
| Perforation | 0 (0) | |
| Decrease of TIMI flow (<2) | 0 (0) | |
| Acute occlusion | 0 (0) | |
| Visible thrombus formation | 0 (0) | |
| Significant air embolus | 0 (0) | |
| Relation to procedure | 0 (0) | |
| Relation to robot | 0 (0) | |
| MACE | 0 (0) | |
| Efficacy endpoint | Procedural technical success | 59 (95.2) |
| Total manual conversion | 3 (4.8) | |
|
Data are n (%). MACE: major adverse cardiac event; NHLBI: National Heart, Lung, and Blood Institute; TIMI: Thrombolysis In Myocardial Infarction
|
||
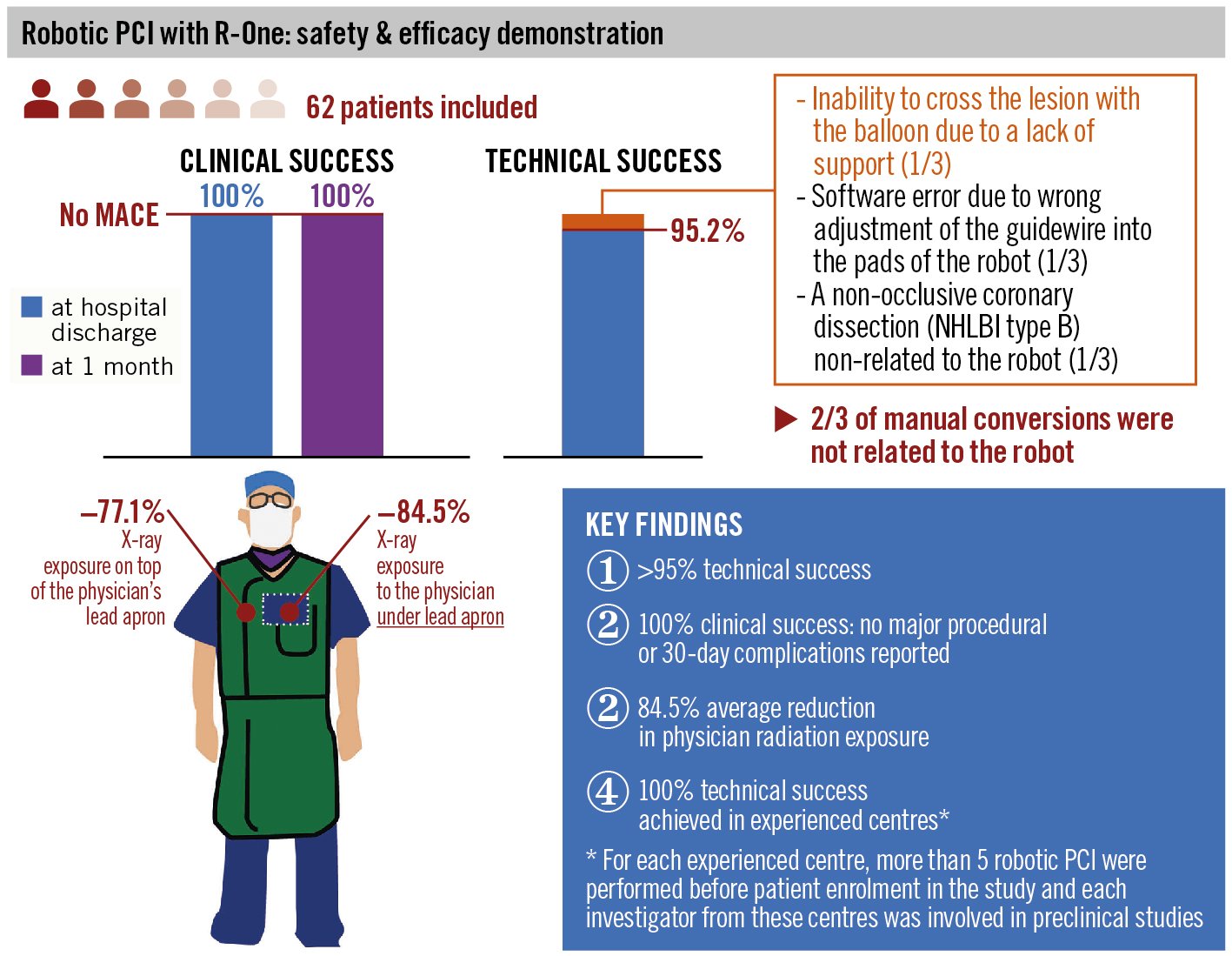
Central illustration. Safety and efficacy of R-One Robotic System for PCI in patients with a de novo coronary artery stenosis. MACE: major adverse coronary events; NHLBI: National Heart, Lung, and Blood Institute; PCI: percutaneous coronary intervention
Table 5. Radiation exposure.
| Variables | Overall population (N=62) |
|
|---|---|---|
| Patient radiation exposure, mGy | 540.3±498.4 | |
| Simulated manual operator radiation exposure, µSv | Total on lead (procedure) | 57.1±61.2 |
| Total under lead (procedure) | 3.2±4.1 | |
| Robotic operator radiation exposure, µSv | Total on lead (robotic) | 7.2±8.7 |
| Total under lead (robotic) | 0.2±0.5 | |
| Operator radiation exposure reduction | Total reduction on lead, % | 77.1±26.1 |
| Total reduction under lead, % | 84.5±25.2 | |
| Data are mean±SD. mGy: milligray; SD: standard deviation; µSv: microsievert | ||
Discussion
The R-EVOLUTION study assessed the safety and efficacy of robotic PCI using the R-One system in de novo coronary lesions and demonstrated high rates of clinical and technical success in a patient population that included 25% complex lesions. Additionally, operator radiation exposure was dramatically reduced compared to manual operation. With an expected increase in PCI procedures over the next several years, the R-One system may enable interventionalists to perform PCI with improved navigation in tortuous vessel anatomy, while reducing their health risks from procedure-related radiation exposure.
Efforts to reduce radiation exposure to interventionalists performing PCI procedures have included guidelines and recommendations from the International Commission on Radiological Protection (ICPR), new generations of imaging systems, lead-free protective gear, and additional forms of lead protection25. Despite these efforts, the catheterisation laboratory remains a high-risk work environment. Over the course of a career, the cumulative radiation exposure to an interventional cardiologist can lead to negative health effects such as cataracts, cancer, and accelerated carotid atherosclerosis1326. A study of radiation exposure during invasive cardiology procedures showed that cardiologists’ heads are exposed to 11–16 times more radiation compared to that received through ambient exposure26. Additionally, orthopaedic complications from the use of heavy lead protective aprons are prevalent1114 and may adversely affect performance and productivity121314.
One of the advantages of robotic PCI is the radiation shield protecting interventionalists from exposure during the procedure, eliminating the need for heavy lead protective equipment which often leads to orthopaedic injuries11121314. Moreover, patients benefit from improved navigational precision and accuracy of wires and devices through tortuous vessel anatomy, leading to a reduction in longitudinal geographic miss (LGM) − cases where the entire length of the injured or stenotic segment is not fully covered by the length of the stent. Patients with LGM often have worse clinical outcomes and increased incidences of major adverse cardiac events (MACE)27. Additionally, with a table position further from the radiation source, radiation exposure is reduced in patients undergoing robotic PCI compared to manual PCI as reported by Patel et al (mGy, median [interquartile range]: 884 [537-1,398] vs 1,110 [699-1,498]; p=0.002 and cGy·cm2, 4,734 [2,695-7,746] vs 5,746 [3,751-7,833]; p=0.003)23.
R-One is a new robotic-PCI system on the market. A preclinical study including 42 porcine coronary stented arteries designed to evaluate the safety and efficacy of the system compared to manual PCI was successful and demonstrated 100% technical success, no MACE, and no significant differences between the 2 groups28. R-One received its CE mark in 2019 and first-in-human procedures were simultaneously performed.
Results from studies of similar robotic systems are detailed in Table 6. In these studies, the reported clinical success was 94.9-100%19212229, technical success was 82.4-98.8%19212229, and in-hospital MACE was 0-0.9%.212229. Thus, the clinical and technical results from the R-EVOLUTION study are similar to the results of previous studies using similar devices. Compared to the R-EVOLUTION study, the PRECISE Study21 had a similar patient population, a comparable prevalence of complex lesions (31.7% in PRECISE; 25.0% in R-EVOLUTION), and a comparable technical success rate (98.8%).
Those results are comparable as CorPath GRX and the R-One are both able to robotically manipulate 1 guidewire and 1 stent balloon and are both fixed to the intervention table.
The CorPath GRX is also able to robotically reposition a guiding catheter through a limited translational distance.
The main difference is the architecture of the motorisation of the guidewire. The CorPath GRX has a motor for the rotation (rotary drive) and a motor for the translation (translation drive). This configuration leads to a different design for the cassette.
The R-One has a unique architecture which is able to combine rotation and translation with a system of pads. This architecture enables a quick manual conversion as the wire is locked into pads (as it would be manually with hands), whereas with the CorPath GRX, all the rotary drive needs to be carefully removed when switching to manual operation.
The contrast volume and robotic procedure times are also comparable, though the R-One system had lower procedure times overall and notably lower patient radiation exposure. Operator radiation exposure was dramatically reduced in both studies, with a median operator radiation exposure of 0.98 μGy and a reduction of 95.2% in the PRECISE study21. This reported median reduction was measured comparing the dose received by the operator at the control station and the dose measured at the procedure table without lead protection, which may result in an overestimation in the reduction of radiation exposure. Additionally, it is unclear whether the dosimeter was activated once the guiding catheter was inserted and positioned manually at the ostium of the targeted coronary. Following the same measurement methodology, the radiation exposure reduction with the R-One system would be 99.6% (0.2 μSv to 57.1 μSv at the procedure table). As the guiding catheter is still positioned manually, the dose received during this step must be considered and explains the absence of a 100% reduction.
Table 6. Results comparison with similar devices.
| Beyar et al29 | PRECISE21 | CORA-PCI22 | Smitson et al19 | PRECISION Registry* | R-EVOLUTION | |
|---|---|---|---|---|---|---|
| System used | RNS | CorPath 200 | CorPath 200 | CorPath GRX | CorPath GRX | R-One |
| Number of sites, n | 1 | 9 | n/r | 1 | 20 | 6 |
| Patients, n | 18 | 164 | 108 | 40 | 980 | 62 |
| Complex lesions, % | n/r | 31.7 | 78.3 | 77.8 | 68.8 | 25.0 |
| Technical success, % | 83.3 | 98.8 | 91.7 | 90.0 | 86.5 | 95.2 |
| Clinical success, % | 100 | 97.6 | 99.1 | 97.5 | 97.8 | 100 |
| MACE, % (follow-up) | 0 (in-hospital) | 0 (30 days) | 0.9 (in-hospital) | n/r | 0 (in-hospital) | 0 (30 days) |
| Total procedure time, min | 44 | n/r | 44.5 | 40.2 | 54.3 | 39.9 |
| Total robotic procedure time, min | n/r | 24.4 | n/r | n/r | n/r | 19.9 |
| Mean fluoroscopy time, min | 8.8 | 11.1 | 18.2 | 17.4 | 17.8 | 10.3 |
| Mean contrast injection volume, mL | n/r | 144.2 | 183.4 | 171 | 118.2 | 118.3 |
| Mean patient radiation exposure, mGy | n/r | 1,500 | n/r | n/r | n/r | 540.3 |
| Mean reduction in operator radiation exposure with lead protection, % | n/r | n/r | n/r | n/r | n/r | 84.5 |
| Median reduction in operator radiation exposure, % | n/r | 95.2 | n/r | n/r | n/r | 100 (under lead)86.07 (on lead) |
| *(Medranda GA, Waksman R. Safety and Efficacy of the Second-Generation Robotic Assisted Systems for PCI. Society for Cardiovascular Angiography and Interventions. 2 July 2021; https://scai.org/safety-and-efficacy-second-generation-robotic-assisted-systems-pci-coverage-late-breaking-science. [Last accessed 7 Dec 2022]). MACE: major adverse cardiac event; mGy: milligray; n/r: not reported; RNS: remote navigation system | ||||||
Study limitations and perspectives
This study was a prospective, multicentre registry and, as such, included a limited number of patients, and only 25% of the treated lesions were complex. The predominant use of a single stent with a low rate of pre- and post-dilatation illustrates low complexity coronary artery disease. In a real-world setting, the technical and clinical success rates may be lower given a more diverse patient population with higher rates of complex lesions. Furthermore, clinical follow-up was limited to 30 days but robotic-induced complications are unlikely to be undetected within the first 30 days as they frequently occur during or immediately after the procedure.
Limitations
At present, robotic-PCI systems have a number of limitations. Manual vascular access and engagement of the coronary artery with the guiding catheter are still necessary. Furthermore, these devices allow manipulation of only 1 coronary guidewire at a time and positioning of only 1 balloon or stent simultaneously. Anatomic or lesion characteristics requiring planned use of any over-the-wire device (e.g., microcatheter, atherectomy) cannot be performed robotically. These limitations will likely be addressed in future generations of robotic-assisted systems.
Conclusions
The R-EVOLUTION study suggests that the R-One system for robotic PCI is safe and effective for the patient while significantly lowering radiation exposure to the operator (Central illustration). Our results indicate that it performs as well as other currently available robotic systems. Given the benefits of robotic PCI, the interventional cardiology standard of care may be redefined without the constraint of modifying the procedural workflow or the devices used, as this system can easily be integrated into any catheterisation laboratory. Further studies are required to evaluate the R-One system in a larger patient population that includes more patients with complex lesions.
Impact on daily practice
The results of our study suggest that R-One is a safe and effective robotic system for performing PCI procedures. With the aid of a robotic system, interventionalists are able to improve the precision and accuracy of guidewire and device navigation through coronary arteries. This system also markedly reduces radiation exposure to the operator while enabling a more ergonomic position, simultaneously reducing the chronic effects of long-term radiation exposure and the orthopaedic complications associated with heavy lead aprons. With a projected increase in the prevalence of coronary artery disease, the need for safe and efficient robotic systems for PCI procedures will probably continue to increase.
Acknowledgements
The authors acknowledge Superior Medical Experts for their editorial assistance, Dosilab for the radiation measurement monitoring, and the European Center for Cardiovascular Research for the study management as an independent Contract Research Organization.
Funding
This study was funded by Robocath SAS.E. Durand has received a grant from the GCS G4 (FHU CARNAVAL).
Conflict of interest statement
R. Sabatier reports compensation from Robocath for support for the present manuscript, consulting fees, training workshops, expert testimony, and participation on a Data Safety Monitoring Board or Advisory Board. E. Durand reports compensation from Robocath for consulting on the present manuscript and from Edwards Lifesciences for consulting on grants and contracts. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

