Introduction
Fluoroscopy-guided interventional procedures are the leading source of occupational ionising radiation exposure for medical personnel1. The prevailing radiation protection measures for interventional personnel include: reduced radiation imaging systems, personal protective clothing, ceiling-mounted shields and table-skirts. However, interventional personnel continue to be exposed to high cumulative doses of X-ray radiation, which may increase the risk for malignancies1, early development of cataracts2, and orthopaedic problems due to the heavy weight of lead aprons3.
Newer dedicated solutions, such as suspended radiation protection systems4 and a remote-controlled robotic system56, provide protection only to the main operator, limit free movement (zero-gravity) and require a significant learning curve (CorPath). The application of a lead-attenuator across the patient's abdomen/pelvis reduces radiation exposure678, but this is limited during femoral access procedures.
The Radiaction Shielding System (RSS; Radiaction Ltd.) was developed to provide full-body protection from scattered radiation to all medical personnel in the interventional suites by encapsulating the imaging beam and blocking the scattered radiation at its origin. It comprises an upper shield around the image detector and a lower shield around the X-ray source, thereby creating a barrier around the imaging beam (Supplementary Figure 1).
The aim of the study was to test the efficacy of the RSS using a phantom for measurements in a clinical laboratory, and to evaluate the feasibility and initial user experience during real-time coronary catheterisation procedures, electrophysiological (EP) procedures and implantations of cardiovascular implantable electronic devices (CIED).
Methods
The shield system
The RSS comprises four main components (Supplementary Figure 1): 1) two robotic extendable shields, upper and lower, composed of discrete overlapping lead-free radiation-blocking segments, assembled on the C-arm around the X-ray tube and image receptor. The device utilises sensors and controls to deploy and retract its attenuating segments and to accommodate C-arm angulation and table movement; 2) a table-mount control panel; 3) indication lights that show the system’s state; and 4) a patient face guard (physical barrier).
Prior to C-arm rotation, the operator retracts the shields to allow undisturbed C-arm motion. Once the C-arm reaches the desired orientation, the shields quickly deploy by extending the telescopic segments and their flexible edges (Supplementary Figure 1). During table panning the RSS can be operated in hover mode, where the segments are partially deployed, thus allowing the table to be freely moved.
Bench tests
The RSS was tested using a phantom for measurements in a clinical laboratory (RANDO anthropomorphic phantom; The Phantom Laboratory). Radiation rates were measured at different locations around the C-arm at two heights (waist and head), at all relevant C-arm angles, and according to X-ray energy levels (nominal and worst-case kV levels). Scattered radiation dose rate measurements were compared in two different setups: without the RSS versus with the RSS deployed (Supplementary Figure 2). In both setups, no additional external shielding was used (no table-mounted drapes, suspended shields etc.).
The scattered radiation was measured by eight sensors: four ion chambers (model 10X6, 1800; Radcal Inc.) and four dose diodes (DDX6-WL solid state low dose sensor; Radcal Inc.). The accuracy for dose and dose rate measurement using the ion chamber or the dose diode is ±5%. The sensors used were designed to work in the range of diagnostic energy levels and low-level radiation doses (Supplementary Appendix 1). The minimum sensitivities of the sensors for bench tests were: ion chamber: 0.01 nSv and solid-state sensor: 0.2 nSv.
Performance assessment in real clinical environment
The RSS was installed for one week in the coronary catheterisation laboratory (CL) and for one week in the electrophysiology (EP) laboratory at Assuta Ashdod University MC. During these two weeks, all procedures were performed with the RSS, except for emergent coronary catheterisations (Supplementary Table 1). This was a non-randomised, non-controlled performance assessment in a real clinical environment. Radiation measurements while using the RSS were compared retrospectively to radiation measurements during a prior week without the RSS. In both setups, standard protection measures were used (i.e., table-mounted drapes and a ceiling-suspended shield). Training was provided for all the staff. Three types of procedures were studied: 1) non-emergent coronary catheterisations; 2) EP procedures; and 3) left-sided CIED implantations.
The sensors' locations in the coronary and EP laboratory and technical details are described in Supplementary Appendix 2 and Figure 1. Sensors #4 (chest height) and #5 (pelvis height) in the CL were placed on the physicians, outside the lead aprons; therefore, they reflect the combined protection of the RSS plus the suspended and table shields. Thermo-luminescent dosimeters (TLD; Soreq Nuclear Research Center) were used for radiation dose measurements (Supplementary Appendix 3). The minimum sensitivity of the TLD sensor for a clinical environment was 0.1 mSv. Average dose rates were calculated by dividing total dose (in mSv) by the total radiation time (sec). Detailed feedback from the users regarding system operation, workflow and safety was obtained.
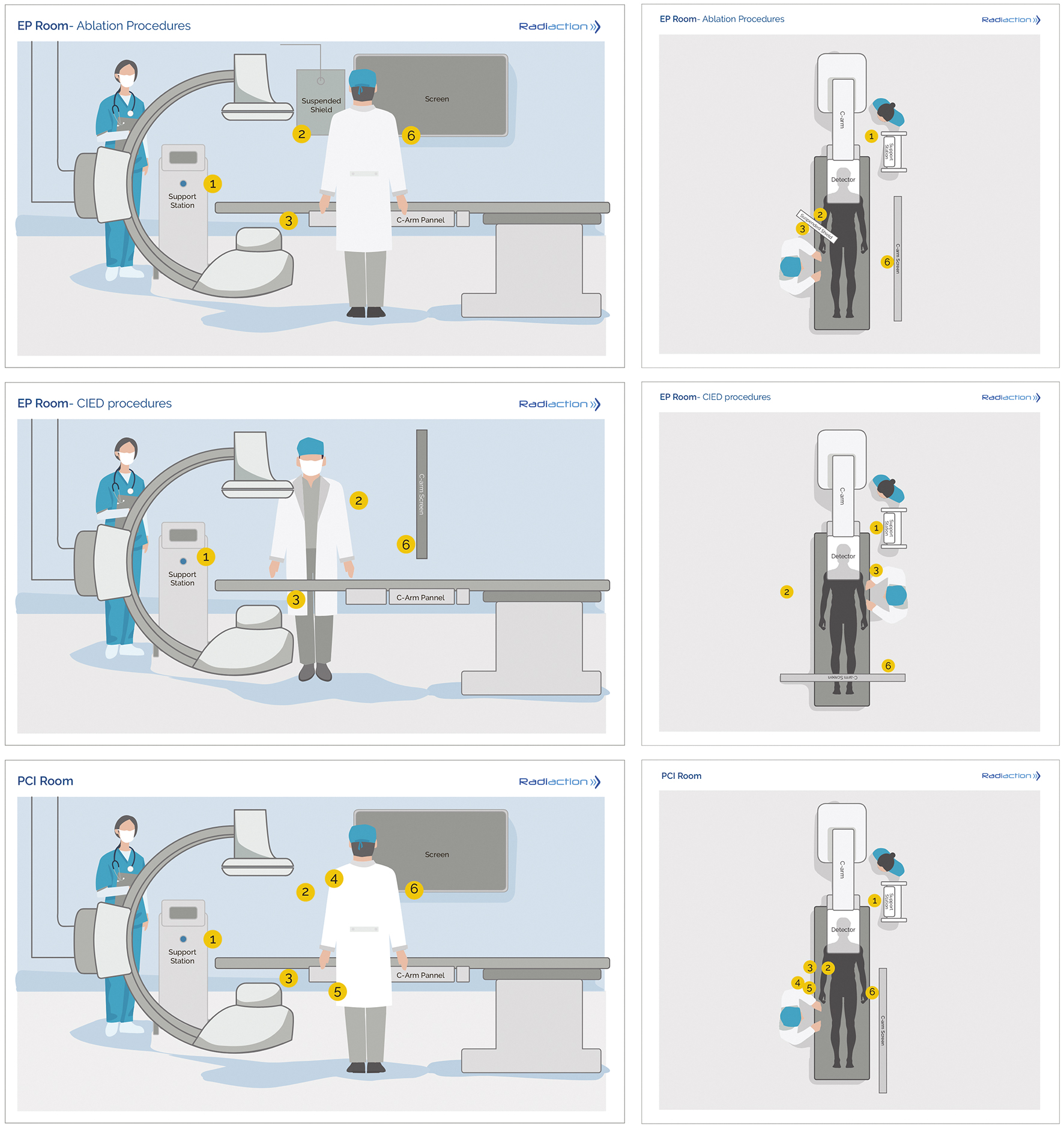
Figure 1. RSS sensor locations. Side view (left) and top view (right) with placement of the six sensors. EP room/ablation procedures: sensor #1: on support system/nurse station, #2: inner side of clear suspended shield (facing C-arm), #3: under the table (inner side of the table mounted drape), #6: on the viewing monitor (chest height); EP room/CIED implantations procedures: sensor #1: on support system/nurse station, #2: inner side of clear suspended shield (facing C-arm, right side of patient), #3: under the table (inner side of the table mounted drape), #6: on the viewing monitor (chest height); PCI room (coronary interventions): Sensor #1: on support system/nurse station, #2: inner side of clear suspended shield (facing C-arm), #3: under the table (inner side of the table mounted drape), #4: on physician chest height outside lead apron, #5: on physician pelvis height outside lead apron, #6: on the viewing monitor (chest height). CIED: cardiovascular implantable electronic devices; EP: emergency procedure; PCI: percutaneous coronary intervention; RSS: Radiaction Shielding System
Statistical analysis
Scatter dose measurements in the bench studies were taken at 38 different C-arm angles. Radiation doses were normalised to exposure time (radiation dose rates). Radiation reduction was calculated in relation to the dose-rate calculations without the shields (Supplementary Appendix 1). In addition, the median reduction for all C-arm angles measured was calculated with a 95% confidence interval (CI).
A non-parametric, Wilcoxon signed-rank test was used for comparison of radiation dose rates, with and without radiation shields, using GraphPad Prism version 9.0.0 (GraphPad Software). Values were paired according to C-arm angulation.
Results
Bench tests
The RSS CL bench tests showed significant radiation reduction performance under full usage of the system, compared to no shielding at all, both in the absence of conventional shielding (93% for main physician - position 1, 94% for second physician - position 4, and an average of 91.5% in all locations) (Table 1, Figure 2, Central illustration, Supplementary Figure 3).
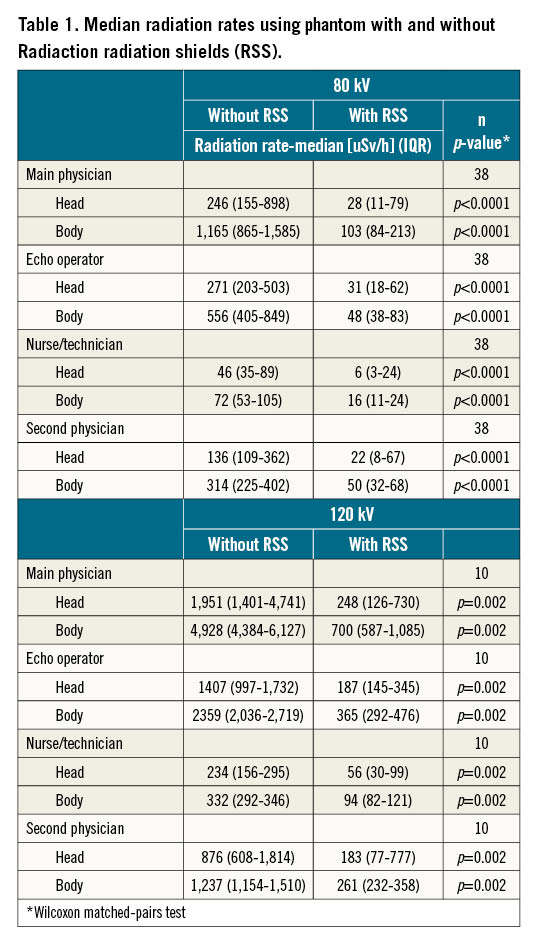
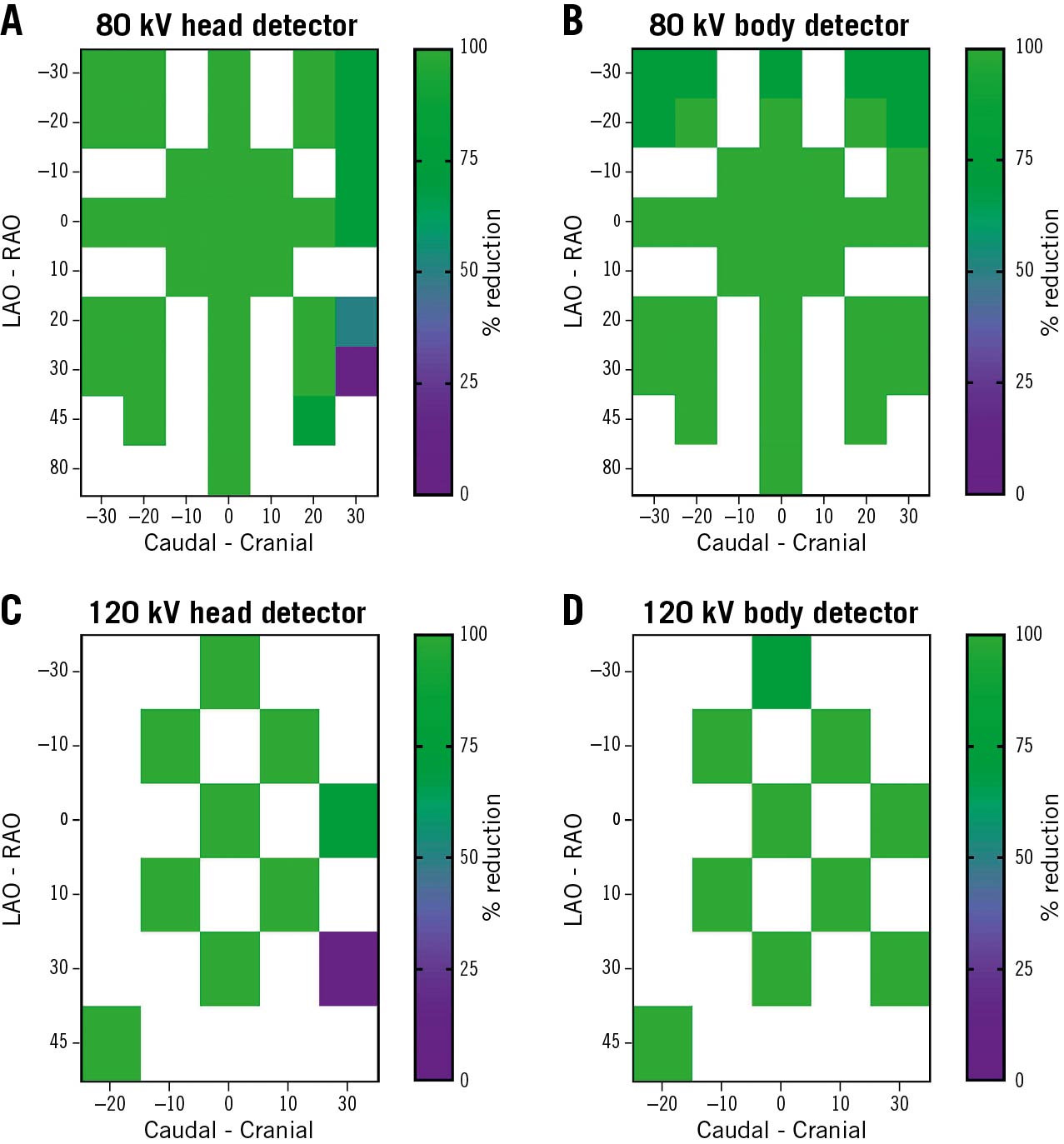
Figure 2. Scattered radiation reduction performance at different CL personnel positions (bench tests). The X-axis represents the C-arm angle in the caudal-cranial plane, the Y-axis represents the C-arm angle in the LAO-RAO plane. A, B) Percentage of radiation reduction (averaged) while using the radiation shields at 80 kV as depicted by the primary operator head detector (height of 155 cm from the floor) and body detector (height of 75 cm from the floor). The colour scale represents the percentage of radiation reduction in 0-25%, 25-50%, 50-75% and 75-100% categories. C, D) Percentage of radiation reduction while using the radiation shields at 120 kV as depicted by the primary operator head detector (height of 155 cm from the floor) and body detector (height of 75 cm from the floor). LAO: left anterior oblique; RAO: right anterior oblique

Central illustration. Mean radiation rate reduction of the robotic Radiaction Shielding System (RSS). Bench tests (left); coronary catheterisation procedures (middle); electrophysiology and CIED procedures (right). CIED: cardiovascular implantable electronic devices; EP: emergency procedure
Clinical environment
Radiation sensors measured total accumulated daily and weekly doses. Average dose rates were calculated according to actual X-ray time, as recorded by the fluoroscopy system (Supplementary Table 2, Supplementary Table 3, Supplementary Figure 4, Supplementary Figure 5). Despite the low procedure sample size, average scatter radiation dose rates were significantly lower with the RSS versus without the RSS in both the EP and the CL at different locations around the C-arm (Figure 1). In the CL, for both upper and lower body sensors of the primary operator (sensors #4,5, Figure 1, Supplementary Table 2, Supplementary Figure 4) with the RSS, the average radiation dose rates were below the detection threshold, compared to 0.13 mSv/h without the RSS. The total weekly radiation time was similar (358.4 minutes without and 384.2 minutes with the RSS). For two ablation procedures, the average radiation rate in all three upper body sensors (sensors #1,2,6, Figure 1, Supplementary Table 3, Supplementary Figure 5) with the RSS was below the detection threshold, and was not available for control ablation procedures (these data were not included in the average calculation). The average dose rate below the table (sensor #3, Figure 1, Supplementary Table 3, Supplementary Figure 5) was 0.232 mSv/h with the RSS and 1.546 mSv/h without the RSS - an 85% reduction. For 10 CIED implantation procedures, the average radiation dose rate in the upper body sensors (sensors #1,2,6, Figure 1, Supplementary Table 3, Supplementary Figure 5) was 0.0055 mSv/h with the RSS and 0.23 mSv/h without the RSS – a 97% reduction. A 77% reduction in average dose rate was measured in the lower body sensor (0.289 mSv/h with the RSS vs 1.257 mSv/h without the RSS). In all CIED implantation procedures, the upper skirts of the RSS above the incision area had to remain retracted to enable the physician’s optimised visualisation of the operation field. The total weekly radiation time for all EP procedures was similar (231.9 minutes without shields vs 181.5 with the RSS). The mean radiation rate reduction over all sensor locations was 91.2±8.9% (range 78.4-100%) in the CL, and 93.3±12.3% (range 77-100%) in the EP laboratory, including CIED implantations (Central illustration, Supplementary Table 2, Supplementary Table 3, Supplementary Figure 4, Supplementary Figure 5).
User experience
Supplementary Table 4 presents the ranking given to a seven-question survey. The feedback showed good satisfaction levels for RSS integration into the procedure workflow and the short learning curve. The satisfaction was less pronounced for the CL team due to limitations and alarms in steep cranial angulations which delayed work flow for a few seconds (with no safety issues).
The feedback showed that the operation of the device is intuitive and simple and does not add significant time to the procedure; the users felt that the RSS was safe, with no perceived changes in image quality. The operators did not notice signs of anxiety or stress in patients concerning the face guard and shields.
Discussion
The RSS is a novel robotic radiation shielding system that provides full-body protection to all medical personnel during fluoroscopy-guided procedures. This is the first study to test the efficacy of this system on a phantom model and to evaluate its feasibility during live interventional cardiology procedures.
Our bench tests showed significant radiation reduction performance under full usage of the system in the absence of conventional shielding, potentially providing 93-94% exposure reduction to physicians in coronary procedures and 87-93% to all medical team members. For the real-world first-in-man use of the RSS, the mean radiation rate reduction was above 90% for all sensor locations in both CL and EP procedures.
User feedback proved the feasibility and ease of use of the system within a short learning curve, with no apparent disturbance to the patient's well-being. The higher ranking in the survey by the EP team may be due to fewer C-arm rotations and the steep cranial angulations required in the CL.
Reducing high radiation exposure during medical procedures has been the principal task of many professional societies and advisory groups910.
The RSS, therefore, has the potential to improve medical team safety by offering radiation reduction to all interventional staff. It provides radiation reduction to the entire body, at all angulations, and appears to integrate smoothly into the clinical workflow. It is recommended, however, to maintain existing standard shielding until additional clinical data are available.
Limitations
Firstly, the first-in-man data were limited and not from a randomised controlled study. Secondly, operators were given the discretion to choose whether to use the RSS or not, and to what degree (fully or partially). This variability may have attenuated the potential benefit from the system. Thirdly, the RSS was not used for emergent coronary procedures, and finally, current sensors might not be sensitive enough in this dose range.
Future studies should measure radiation exposure per specific procedures using more sensitive sensors, employ a controlled randomised design, and a large number of procedures should be performed beyond the operator’s learning curve. Further studies and improvements of the system may potentially lead to a reduction in the need for personal protective clothing.
Conclusions
This first report of the RSS bench testing and performance in a live clinical environment shows a significant reduction (but not elimination) of radiation and a high level of integration in the clinical workflow, as well as a good safety profile for both coronary and EP procedures. Thus, the RSS may have an important role in both full-body and whole team protection from scattered radiation during interventional cardiology procedures. Until more data are available to show elimination of radiation by the RSS, it is recommended to maintain existing standard shielding.
Impact on daily practice
Current standard radiation protection fails to fully protect interventional cardiology personnel from scattered radiation, leaving the head, hands and feet exposed. A novel robotic radiation protection device was developed to provide full-body protection to all medical personnel by encapsulating the imaging beam and blocking scattered radiation. Bench tests show significant radiation reduction, and preliminary clinical evaluation shows the system is safe and highly integrated into the clinical workflow.
Conflict of interest statement
E. I. Lev and A. Finkelstein received payments as clinical advisors for Radiaction Ltd.. A. Finkelstein serves as a proctor and consultant for Medtronic and for Edwards Lifesciences. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

