
Abstract
Aims: This study aimed to evaluate the effectiveness of ceiling suspended screens, lead glasses and lead caps in reducing radiation doses to the brains of interventional cardiologists.
Methods and results: Interventional procedures where the thorax of the patient is irradiated with different beam projections were modelled. The dose reduction in the white matter and hippocampus of the Zubal head phantom was studied for two sizes of ceiling suspended screens, two types of lead glasses and lead caps of surgical and hood models, which cover different regions of the head. Ceiling screens were the most effective, reducing the dose to brain tissue by 74% or even as much as 94%. The dose reduction provided by lead glasses varies between 10% and 17%. For the lead caps, it strongly depends on the model, varying from 6% (surgical) up to 68% (hood that also covered lower parts of the head).
Conclusions: The dose to the brain can be reduced by using appropriate radiation protection devices. This study has shown that lead caps are less protective than previously described and that the best protection is given by ceiling suspended screens, which are widely available in interventional theatres.
Abbreviations
DB/C: percentage of the dose at the chest level received by the brain tissue (white matter or hippocampus)
DR: percentage dose reduction
Hood-SF: hood with shielded forehead
Hood-UF: hood with unshielded forehead
IC/IR: interventional cardiologists and radiologists
SS: side shielding
Introduction
Compared to other organs and tissues, such as the colon, lungs and red bone marrow, the adult brain is understood as one of the lowest radiosensitive tissues, due to the post-mitotic condition of its cells1. However, a number of recent studies have reported the occurrence of brain tumours in healthcare professionals who are chronically exposed to medical X-rays (interventional cardiologists and radiologists)2,3 and indicated a twofold increased risk for brain cancer mortality compared to unexposed controls4. Interventional cardiologists are acknowledged to receive high occupational doses of scattered X-ray radiation5. Such reports have raised the concern that a link between chronic exposure to scattered X-rays and side effects in the brain may exist, suggesting a higher radiosensitivity than currently thought.
An intriguing fact about the reported lesions relates to their laterality (occurrence in the left or right hemisphere). Roguin et al3 presented 31 brain and neck tumours in interventional cardiologists and radiologists (IC/IR) and two other specialties. Anatomic localisation of the tumours was possible in 26 cases, of which 22 were on the left side (85%), usually the most exposed side of the operator’s head. Besides brain tumours, cognitive impairment has been reported by Marazziti et al6. In a neuropsychological test performed with exposed and non-exposed staff, impaired verbal long-term memory was observed in the former group, an ability that is modulated mainly by the left hippocampal hemisphere6.
However, in studies not related to ionising radiation seeking to map the laterality of brain tumours, a preferential side of brain lesions could also be observed, even though it varied between studies. Ellingson et al7 reported a higher incidence of glioblastomas on the left side of the brain, whilst Larjavaara et al8 observed a major occurrence of gliomas in the right hemisphere (51%). The laterality can be due to several factors, namely genetics, age at occurrence of the lesion, extracellular environment, metabolism, etc.8. Therefore, the laterality observed in the brain lesions reported in IC/IR could have had an origin other than the exposure to radiation.
It is also interesting to mention that, contrary to the assumed low radiosensitivity of the brain, in cohorts of nuclear/uranium cycle workers, with cumulative external whole body doses in the range of 1-500 mSv9, a higher standardised mortality ratio due to brain tumours was observed9-11, despite the fact that an excess relative risk was not observed. In addition, low doses seem to play a role in the development of benign tumours in the nervous system and pituitary glands12, and the mechanisms at the cellular and molecular level have been reported to respond differently in high or low dose ranges13.
Although no conclusive connection has yet been made between chronic exposure to low doses of X-rays and side effects in the adult brain, there has been an increasing interest in protection devices that offer shielding to the head14-16. For this reason, the aim of this study was to evaluate, by computational methods, the effect of currently available shielding devices (ceiling suspended screens, lead glasses and lead caps) in reducing the dose received by the brain of the operator during cathlab procedures. Focus is given to the white matter and hippocampus, because these structures are of concern in the development of lesions and cognitive impairment6,7. Furthermore, the dose reduction in the brain protected by the lead caps was compared to the dose reduction measured by detectors placed on the head of the operator underneath the caps, in order to assess the correlation between both.
Methods
Monte Carlo calculations are well established and validated methods for radiation dosimetry17. In this study, the energy deposited in the brain of an interventional cardiologist performing an interventional procedure in which the thorax of the patient is irradiated was calculated using tally f6 of the Monte Carlo code MCNPX18. Anthropomorphic mathematical phantoms were used as patient and operator models19. The operator head was simulated in greater detail by the Zubal phantom, including the hippocampus and white matter20. This phantom is composed of soft tissue, muscles, bones, blood, fat and skin21. The left and right sides of the hippocampus and white matter tissue were segmented separately using imaging processing software22. The operator model was equipped with a 0.5 mm thick lead apron and thyroid collar in all simulations.
A primary X-ray beam of 80 kVp filtered with 3 mm Al was projected towards the thorax of the patient. The distance from the source to the skin of the patient was 60 cm and the diameter of the field size at the entrance of the flat panel detector, placed 10 cm away from the skin of the patient, was 25 cm. The operator was positioned for a brachial access (40 cm away from the centre of the beam) on the right side of the patient (Figure 1). These parameters were kept constant in the simulation of six common beam projections: anterior-posterior (AP), posterior-anterior (PA), left and right oblique at 45° and 90° (LAO45, RAO45, LAO90, RAO90).
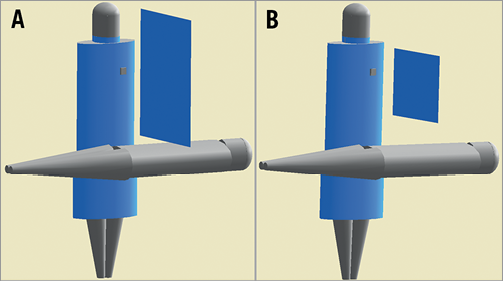
Figure 1. Operator, patient and ceiling screen positioning in the simulations. A) Large ceiling screen positioned 1 cm from the patient and B) small ceiling screen positioned 15 cm from the patient
The influence on absorbed radiation dose in the white matter and hippocampus was evaluated when ceiling suspended screens, lead glasses and lead caps were used. All devices were modelled as composed from pure lead and their efficiency was evaluated individually (only one device was simulated at a time). Small (40×50 cm) and large (61×76 cm) 0.5 mm thick ceiling suspended screens were considered and modelled at 1 cm and 15 cm above the patient. Two models of lead glasses were considered with frontal lenses of 0.75 mm. One model had a wraparound style while the other had flat frontal lenses and additional 0.5 mm side shielding. Finally, three models of commercially available lead caps of 0.5 mm thickness were modelled (one surgical and two hoods) (Figure 2). The surgical model is 12 cm high and covers the head obliquely from above the eyebrows and the nape of the neck. The hood models cover all around the head; the main difference between them is the area of the head that is unshielded. For the unshielded forehead type, hood-UF, the area below the nose up to the top of the forehead is unshielded, whilst for the shielded forehead type, hood-SF, the forehead is also shielded, with the area from the thyroid collar up to above the eyebrows unshielded.
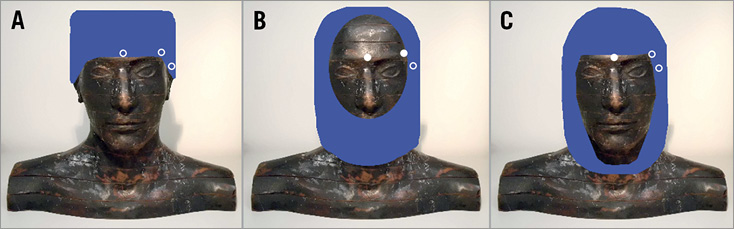
Figure 2. Three models of lead cap were evaluated: (A) surgical, (B) hood-UF and (C) hood-SF, each one offering shielding to different parts of the head. The white circles illustrate the position of the detectors placed on the skin of the phantom. Detectors illustrated as open circles were shielded by the cap, whilst solid circles indicate that the detector remained unshielded.
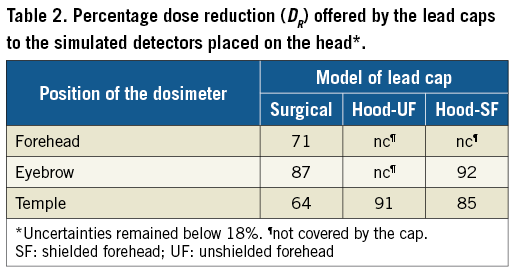
Dose reduction of lead caps is typically reported by transmission measurements using dosimeters placed below them14,16. In order to compare the values obtained by this approach with the dose reduction in the brain organ itself, three detectors (0.16 cm³) made of soft tissue material were simulated on the left temple, on the forehead between the eyes and at the end of the left eyebrow (ipsilateral to the beam). The position of all three detectors on the head was 1 cm higher than the lower border of the surgical cap, thus completely shielded. At these locations, hood-UF did not shield the detectors on the forehead and eyebrow, whereas hood-SF did not shield the detector on the forehead.
The percentage dose reduction DR granted by the protection devices to the brain tissue and dosimeters on the skin of the phantom was calculated as the difference between the dose without (Dwithout) and with shield (Dwith), divided by the dose without shielding (Dwithout), expressed as follows:
![]()
In addition, in order to trace the origin of the radiation delivering the dose to the brain, the Zubal phantom was replaced by a simplified head, with the brain defined as a single organ (without distinction between white matter and hippocampus, nor left and right hemispheres). Four regions around the head were defined by sagittal and frontal planes (Figure 3A): ipsilateral anterior (a), contralateral anterior (b), contralateral posterior (c) and ipsilateral posterior (d). These regions were further split transversally into three sections of the same size, each comprising where the jaw, eyes or forehead would be (Figure 3B). The neck was also separated according to the four regions defined. By using cell flagging in the Monte Carlo code, every photon depositing energy in the brain could be tracked to identify via which of the sections it entered the head before hitting the brain. Thus, the contribution to the dose in the brain from each of the considered sections was traced.
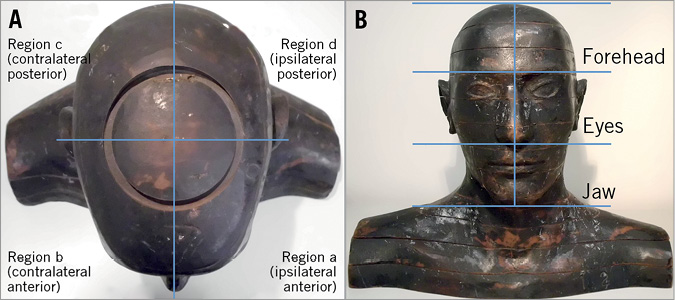
Figure 3. The head of the operator was divided into (A) regions a, b, c and d, and (B) sections comprising the forehead, eyes and jaw. The contribution to the dose in the brain tissue from radiation crossing each section was assessed.
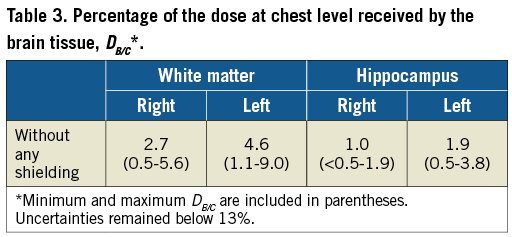
Finally, in order to compare the dose in the white matter and the dose in the hippocampus to the dose measured routinely at chest level, a radiation badge was defined as a small slab of soft tissue, 4×4 cm and 10 mm thick23, placed on the left side of the operator’s chest, over the lead apron. The ratio DB/C, in percentage and defined as
![]()
was evaluated for the unshielded brain.
Results
Table 1 shows the radiation dose reduction in the brain, according to the presence of each protection device. Ceiling suspended screens provided the best protection, decreasing the dose by from 74% (at the hippocampus) up to 94% (at the white matter).
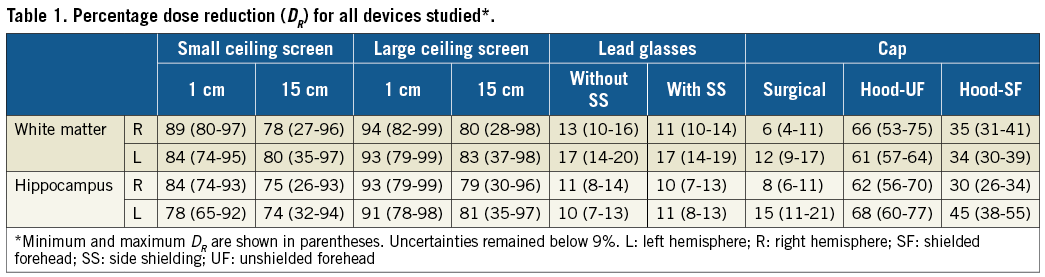
Lead glasses are able to provide some shielding to the brain, in addition to their value for eye lens protection24, being equally efficient regardless of the presence or absence of side shielding. If only lead glasses are used as protection, the dose to the brain can be reduced by between 10% and 17%.
The protection granted by lead caps depends substantially on the model. The surgical model provides the poorest shielding, decreasing the dose by only between 6% and 15%, comparable to the dose reduction obtained by using lead glasses. Although hood-UF exposes a larger area of the forehead compared to hood-SF, it reduces the dose in the brain by more than 50%, whereas the dose reduction with hood-SF was never higher than 55%.
For the simulated detectors positioned on the head of the phantom (Table 2), the superficial dose reduction offered by the caps varies from 64%, for the detector at the temple, covered by the surgical model, up to 92%, for the detector at the eyebrow shielded by the hood-SF.
Without any protection device, about 90% of the radiation contributing to the dose in the brain strikes the head at region a (ipsilateral-anterior to the beam), with the major amount (more than 80%) coming from the jaw and eyes sections (Figure 4). Only 5% crossed the head in the forehead section and only a minor contribution reached the brain from the neck, which was protected by the thyroid collar. Radiation reaching the head in all sections of regions b, c and d contribute, altogether, to less than 10% of the dose in the brain.
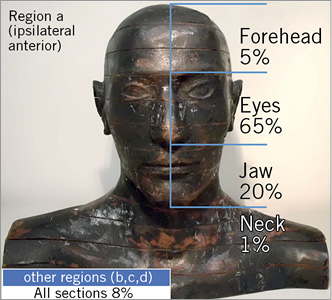
Figure 4. The highest contribution originates from the ipsilateral anterior region (a), eyes and jaw sections. Results are presented as average over AP, PA, RAO90 and LAO90 projections. Uncertainties remained below 6%.
The dose to the brain, without any shielding, compared to the dose in the dosimeter at the chest level (DB/C) varied from less than 0.5% (LAO90), in the right side of the hippocampus, up to 9%, in the left hemisphere of the white matter (RAO90) (Table 3), depending on the beam projection. Averaged over six beam projections and taking into account both the white matter and hippocampus, DB/C remained lower than 5%.
Discussion
This study evaluated the impact of several radiation protection devices on the absorbed radiation dose to specific tissues inside the brain (hippocampus and white matter) of interventional cardiologists. In addition, it assessed the origin and trajectory of the radiation that delivers this dose. To the best of our knowledge, this is the first study to have reported such data.
Our results demonstrate that ceiling suspended screens and lead caps of the hood model (Figure 1B) provide the best protection to the brain. The high effectiveness of a ceiling suspended screen to protect the operator, as has been pointed out previously23, applies to the brain tissue as well, providing the highest dose reduction among the devices evaluated. Both sizes of suspended ceiling screen were most efficient with the primary beam pointed horizontally towards the operator (RAO90). Conversely, the lowest dose reduction in the brain occurred with the primary beam below the couch (PA). In this specific beam projection, the gap between the ceiling suspended screen and the patient is of critical importance. For example, the dose reduction granted by the large ceiling suspended screen in PA projection goes from 82% when close to the patient to only 28% when shifted 15 cm above the patient. Nevertheless, keeping the primary beam under the couch results in lower doses to the medical staff25,26 and, by placing the ceiling suspended screens close to the patient, the radiation dose to the eye lenses and extremities of operators can also be reduced23,25,27.
In contrast to previous studies15,28, we observed that lead glasses do provide some protective shielding to the brain. The apparent contradiction with the other studies can be understood by evaluating the uncertainties reported by Marshall et al28 for measurements performed in an 80 kVp beam and the evaluation of the dose reduction provided by the glasses in a similar set-up (AP projection and averaged over both sides of hippocampus and white matter). Whereas the uncertainties are estimated to be 20%, the dose reduction attains 10%. This result also indicates the higher sensitivity of numerical models in identifying such small variation. The small protection offered by lead glasses, in contrast to the high contribution from the eye section to the dose in the brain tissue, as presented in Figure 4, relates to the small size of the lenses and their distance from the head, which does not suffice to offer proper shielding.
The main source of radiation to the medical staff in interventional cardiology is the patient, in whom the primary beam is scattered in a complex, non-uniform manner29. Therefore, the radiation contributing to the dose in the brain reaches the head of the operator mostly from below, obliquely, as shown in Figure 4. The substantial difference in shielding provided by the different models of lead cap is directly related to this observation. The regular surgical model offers almost no coverage to the eyes and jaw sections and is, therefore, not very efficient in shielding the brain. Indeed, this is confirmed by comparing the amount of radiation reaching the head from the forehead section of region a (5%) (Figure 4), and the percentage of dose reduction provided by the surgical cap (10%, averaged over all structures) (Table 1). The same applies to hood-SF: although it covers most of the forehead and offers a better shielding than the surgical model, a large area in the lower part of the head remains unshielded. In contrast, the hood-UF model, although with the forehead mostly unshielded, provides the highest dose reduction to the brain, owing to the shielding of the jaw section. These findings agree with a previous study15, where improved shielding to the brain was provided by a hood cap compared to the surgical model. Notwithstanding, the dose reduction reported by Fetterly et al15 with a hood cap unshielded at the chin is higher than the values found in our study, because of the different geometries considered (primary beam vs. scattered beam, respectively).
A much higher dose reduction was observed in the detectors placed underneath the cap on the head of the phantom compared to the reduction in the brain tissue for all models of lead cap. Overall, the dosimeters placed under the cap of the surgical model showed a lower dose reduction compared to the other models of cap, most likely because of backscatter from regions of the head that were left unshielded. Amongst all positions of the detectors considered, none proved to be suitable for the estimation of the dose reduction experienced by the brain tissue. Notwithstanding, our results concerning the dose reduction in the detectors showed good agreement with reported data obtained from measurements during clinical practice. Uthoff et al30 found an attenuation of more than 70% for two caps of the surgical model of different materials. This was verified by a detector in a position equivalent to the eyebrow detector reported in our study, for which a dose reduction of 87% was observed (Table 2). Also for a non-lead surgical cap, Alazzoni et al31 found an attenuation of around 80% at the left temple, whilst in our study, in a similar position, a reduction of 64% was obtained. Karadag et al14 evaluated the shielding granted by a hood cap, similar to the hood-UF modelled in this present study, with a dosimeter at the temple of the operator, obtaining a dose reduction higher than 96%. In the same context, we found a dose reduction of 91%. Such agreement indicates that the results of the brain dose reduction obtained in our study may well be expected in clinical practice. Nevertheless, it also indicates the need for caution when assessing the shielding efficiency of lead caps by placing dosimeters underneath them, because the results can give a misleading feeling of protection. In reality, the dose reduction to brain tissue is much weaker, mainly caused by the direction of the scattered radiation that reaches the head.
Reported radiation doses received by interventional cardiologists vary greatly, due to a number of factors: experience of the operator, complexity of the interventions, type of X-ray equipment and dose reducing tools, workload, the use of personal protection devices and position of the radiation badge32-34. As a result, estimations of annual doses received by the brain tissue are not straightforward. Nonetheless, our study may provide a basis for estimating the doses delivered to the brain of physicians if a dosimeter is worn over the lead apron. Using such an approach, the doses received by the brain tissue were shown to be between 20 and 100 times lower than the doses received at the chest level. However, further studies seeking a better way of estimating the dose in the brain tissue by using dosimeters are necessary.
Limitations
The operator phantom was, of course, static during the simulations and its head was always orientated in a forward direction. The dose reduction granted by the protection devices will be affected when the head of the operator has another orientation. This study considered only the irradiation of the patient’s thorax. A procedure where the head or abdomen of the patient is irradiated, or a different access route is used, will change the relative position of the operator with respect to the radiation field and could influence both the shielding efficiency of the protection devices and DB/C, as well as the contribution from the different sections of the head to the dose in the brain.
Conclusions
In the present study, we evaluated the radiation dose reduction in the white matter and hippocampus for interventional cardiologists afforded by different types of protection device. Ceiling suspended screens and lead caps of the hood model offering shielding to the lower parts of the head were the most effective. In addition, our study indicated that the dose reduction measured directly under a lead cap severely underestimates the dose to the brain tissue.
| Impact on daily practice Protection devices commonly found in interventional theatres offer radiation shielding to the brains of interventional cardiologists. Suspended ceiling screens, if properly used, are the most effective. Lead glasses also contribute to the shielding of the brain and lead caps of hood models provide higher shielding than surgical models. |
Funding
This study was partially supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasilia, Brazil, process number BEX 0961/13-2 to EHS.
Conflict of interest statement
The authors have no conflicts of interest to declare.

