Medical radiation from x-rays and nuclear medicine is the largest manmade source of radiation exposure in western countries, and accounts for a mean effective dose of 3.0 milliSievert (mSv) per head per year, equivalent to the radiological risk of 150 chest x-rays1,2. Of these, 0.43 mSv come from interventional radiology (0.20 mSv) and interventional cardiology (0.23 mSv). Amongst adult cardiology patients, fluoroscopically-guided diagnosis and intervention account for 12% of all radiological examinations performed, and 48% of their total collective dose3. On average, a diagnostic invasive coronary angiogram corresponds to a patient radiation exposure of about 7 mSv (range 2-16), while coronary stenting corresponds to 15 mSv (range 7-56)4. Progressively higher effective doses are observed for transcutaneous aortic valvuloplasty, dilation of chronic total occlusion of coronary arteries and endovascular thoraco-abdominal aneurysm repair procedure (Figure 1A). Operator dose per procedure can vary widely, and correlates somewhat with the patient dose – but 1,000 times lower (with the order of magnitude in microSievert, µSv, rather than mSv)5,6 (Figure 1B). Most experienced (and most exposed) interventional cardiologists have an exposure per annum two to three times higher than that of radiologists7, with a typical cumulative lifetime attributable risk on the order of magnitude of one cancer (fatal and non-fatal) per 100 exposed subjects8. However, adequate radioprotection training and diligent protection can reduce the radiation exposure by 90%9. The International Atomic Energy Agency recently launched a campaign to increase the justification and optimisation of radiological examinations through the “3A’s” strategy: “Appropriateness, Audit and Awareness”10. Several articles recently published in EuroIntervention fit well within the framework of this strategy, in particular focusing on Audit and Awareness.
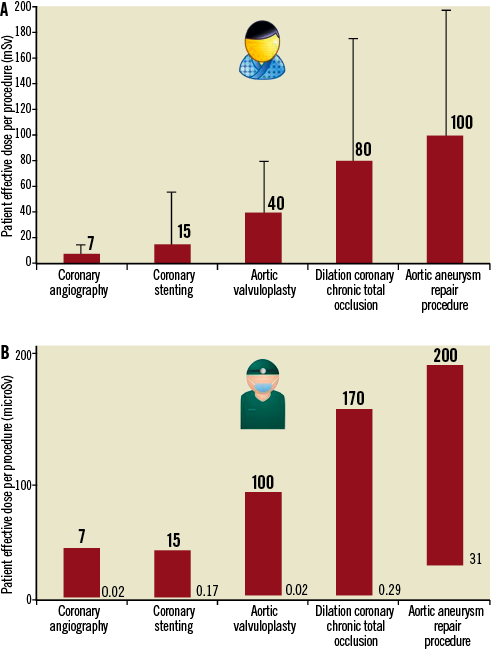
Figure 1. A) Average reference dose (and range) of effective dose of patient per single procedure (modified from references 4 and 5). B) Reference dose (and approximate range) of effective operator dose per single procedure (modified from references 5 and 6). Note the different scale on the y-axis, with microSievert (µSv) units.
Audit of doses
Patient dose is usually measured as Dose-Area Product (DAP) or Kerma-Area Product and can be roughly translated into mSv with a conversion factor (in adults: DAP×0.20=mSv for cardiac procedures). Some variables determining the dose are beyond operator control, and therefore a high DAP is not necessarily associated with an inappropriate procedure. Sometimes, both the operator and the patient have a “blind date” with the dose11. Daneault et al defined the TAVI dose, emphasising that the patient doses are lower with the transapical as compared with the transfemoral approach12. In theory, we need to know what we do to our patients (and to ourselves). Before the procedure, the radiation risk should be part of the informed consent form. After this, the actual delivered dose should be recorded and included in patient records13. The absence of a systematic dose audit impacts not only on the patient but also on operator safety. The WIN study reports that in a “real world” situation 7% of respondents never wear a radiation badge for monitoring purposes and only 66% regularly review their own exposure data14, making it difficult to deploy an effective radioprotection strategy: health physicists are there to help reduce the radiation exposure “fever”, but the patient refuses the thermometer.
Awareness of risks
A highly effective, and possibly the best, way to improve radiological awareness within the cardiology community is to involve cardiologists in studies evaluating the health effects of radiation on themselves. There are radiation-induced non-cancer effects, such as eye cataracts, which can be observed in one-third of staff after 30 years of work15. Buchanan et al describe a self-reported 2% prevalence of cataracts in exposed staff14, but the prevalence is likely to increase substantially if a specific ophthalmology check is advised. There are also radiation-independent health effects, due to logistics and protection, such as the high prevalence of orthopaedic disorders associated with years of work as reported by Buchanan et al14. X-rays are a proven carcinogen, and the major occupational hazard is cancer. The recent report in this Journal of a cluster of cases of brain cancer (mostly left-sided, consistent with the higher left-side exposure of the invasive cardiologist)16 and the higher prevalence of cancer in female radiation workers (who are 38% more sensitive than males to radiation effects) by the Women In Innovation (WIN) group14 are important contributions to the definition of the risk profile of interventional cardiologists and staff. In the present era of evidence-based medicine, case series and retrospective registry data may be the weakest level of evidence, but they often remain the first line of evidence. Further data will soon be available from two ongoing studies: the North-American Multispecialty Occupational Health group supported by the National Institute of Health and National Cancer Institute6 and the Italian Healthy Cathlab study, organised by the Institute of Clinical Physiology of the National Research Council with the Italian Society of Invasive Cardiologists17.
Conclusions
Decreasing patient dose will result in a proportional decrease in scatter dose to the operator, with a substantial reduction of the victims of radiological “friendly fire” among interventional cardiologists and staff. Attention to radiation protection is one aspect –and not the least important– of good practice of medicine. A smart interventional cardiologist cannot be afraid of radiation, but must be very afraid of radiation unawareness.
Conflict of interest statement
The authors have no conflicts of interest to declare.

