Abstract
Concerns regarding radiation exposure and its effects during pregnancy are often quoted as an important barrier preventing many women from pursuing a career in Interventional Cardiology. Finding the true risk of radiation exposure from performing cardiac catheterisation procedures can be challenging and guidelines for pregnancy exposure have been inadequate.
The Women in Innovations group of Cardiologists with endorsement of the Society for Cardiovascular Angiography and Interventions aim to provide guidance in this publication by describing the risk of radiation exposure to pregnant physicians and cardiac catheterisation personnel, to educate on appropriate radiation monitoring and to encourage mechanisms to reduce radiation exposure. Current data do not suggest a significant increased risk to the fetus of pregnant women in the cardiac catheterisation laboratory and thus do not justify precluding pregnant physicians from performing procedures in the cardiac catheterisation laboratory. However, radiation exposure among pregnant physicians should be properly monitored and adequate radiation safety measures are still warranted.
Introduction
Over the past decade, percutaneous coronary intervention (PCI) has increased by 58%, with an estimated 1.3 million PCI procedures now performed annually in the United States1. These procedures continue to increase in anatomic and technical complexity requiring greater fluoroscopy time and subsequent radiation exposure to the patient and catheterisation laboratory personnel2. Occupational radiation exposure is of importance to all members of the cardiac catheterisation team as this has the potential to increase the risk of malignancies and other health hazards3.
Women find this risk of even greater concern during child bearing years as radiation exposure is listed as a reason for altering a career plan in cardiology to a minimally exposed field in 24% of women4. According to the American Association of Medical Colleges women now account for 49% of all medical students and 44% of all internal medicine residents5. However, only 18% of cardiology fellows are women, with only 8.7% in interventional cardiology fellowships4. The proportion of women who choose interventional cardiology as a career is less than half of the rate of women going into general surgery, and currently only 5.9% of board certified interventional cardiologists are women6. Even when women do not choose a career in interventional cardiology, radiation exposure during pregnancy may be an issue while completing fellowship. In addition, female cardiac laboratory nurses and radiology technicians may have concerns regarding their risk with pregnancy. For women to make informed decisions, a clear understanding of the risk of radiation exposure during pregnancy including risk to the fetus is required. Understanding the magnitude of the risk and mechanisms to limit radiation exposure are critical.
Risks and concerns specific to the foetus
Radiation exposure to the embryo or foetus could lead to two types of adverse effects: deterministic and stochastic effects. Deterministic effects result from damage to a number of cells for which there is a threshold before any clinical effects happen. The main deterministic effects in the developing embryo or foetus consist of intrauterine growth retardation, pregnancy loss, mental retardation, small head size, reduced intelligence quotient (IQ) and congenital malformations. Stochastic (random) effects result from damage to single cells for which there is no threshold but there is an increased probability of these effects as the radiation dose increases. The main stochastic effects from radiation exposure to the embryo consist of childhood risk of cancer and hereditary diseases in the descendants7,8. The development of these effects depends on the age of the conceptus when the radiation exposure occurs and the amount or the dose of radiation to which it is exposed.
The biological effects of radiation are at the deoxyribonucleic acid (DNA) level which may result in three outcomes: (1) injured or damaged cells repair themselves resulting in no residual damage; (2) cells die; or (3) cells incorrectly repair themselves resulting in biological changes that could lead to the development of cancer and genetic defects among children of parents exposed to ionising radiation9,10. Biomarkers, such as the test of chromosomal aberrations in peripheral blood lymphocytes, demonstrate that high frequency of chromosomal breakage is a strong predictor of cancer risk in healthy subjects11,12.
Probability of healthy children being born
The primary risk for a pregnant worker’s child is cancer induction. Wagner and Hayman estimated the overall probability that a child will suffer a malformation or cancer assuming a normal incidence of childhood cancer is ~0.07% (Table 1)14,15.
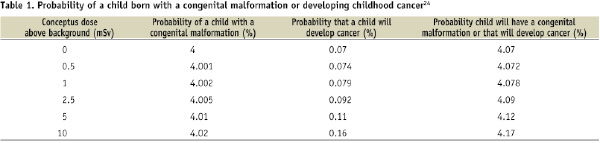
The predicted probability of a live birth without malformation or cancer is reduced from 95.93% to 95.928% following conceptus exposure of 0.5 mSv, using a conservative estimate from the NCRP. Exposures above 10 mSv were predicted to increase the risk by 0.1%. However, it is possible that there is no added risk at all.
Actual radiation exposure to the foetus
No data are currently available which adequately demonstrate the actual radiation exposure to the foetus in women working in the cardiac catheterisation laboratory. However, to analyse the risk we evaluated the data from the Mayo Clinic, Rochester, MN, USA in all women regardless of profession and in any clinical area who wore a pregnancy radiation badge. Of the 68 women where we had matching collar and waist radiation badges, 56 (82.4%) had an undetectable radiation measurement from the badge under the lead at waist level including one interventional cardiologist and an interventional cardiology fellow (Glenn M. Sturchio, personal communication). Of the remaining 12 women who did not have undetectable radiation levels, nine were nuclear medicine technicians or nurses, two were X-ray technicians, and another worked in anaesthesiology. The increased radiation exposure could be explained by the fact that nuclear medicine technicians and nurses do not routinely wear lead aprons for protection.
Dose monitoring and radiation dose assessment
Dose limits
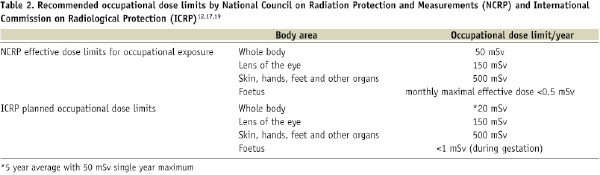
To understand dose limits, one must understand what is meant by tissue dose (absorbed dose) which is expressed as Gray (Gy) or effective dose which is expressed as Sievert (Sv). Modern x-ray systems commonly report the procedure-cumulative kerma-area product (KAP, Gy·cm2). KAP is the literal product of air kerma (kinetic energy released in material which is the sum of the initial kinetic energies of all charged particles liberated by uncharged ionising radiation in a sample of matter, divided by the mass of the sample) and the x-ray field area at the location of the interventional reference point and describes the total x-ray energy incident upon a patient. With appropriate conversion factors, KAP values can be used to estimate skin dose-area product (DAP, Gy·cm2) and patient effective dose. The effective dose is an estimate of the uniform, whole-body equivalent dose that would produce the same level of risk for adverse effects that results from the non-uniform partial body irradiation and is a calculated dose. In general, the foetal dose of radiation is often described as a tissue dose, although this is not always uniform. The National Council on Radiation Protection and Measurements (NCRP) recommends limiting occupational radiation exposure of the foetus to a value as low as is reasonably achievable (ALARA) but not to exceed 5 mSv (500 mrem) during the entire pregnancy and 0.5 mSv per month of the pregnancy16. The risk of induced miscarriages, malignancies, or major congenital malformations in embryos or foetuses exposed to doses of ≤50 mGy is negligible compared with the spontaneous risk in those without radiation exposure17. A report from the American College of Obstetricians and Gynaecologists supports the recommendation from the National Council on Radiation Protection and Measurements and states that pregnant women exposed to radiation dose ≤50 mGy (5 rad) have not been associated with an increase in foetal anomalies or pregnancy losses18. Generally fetal radiation below 50 mSv (5 rem) is considered negligible7. This is based on studies demonstrating that exposure to a cumulative dose of less than 50 mGy (5 rads) during pregnancy does not affect the outcome of the pregnancy compared to control populations exposed to background radiation estimated as less than 1 mGy (0.1 rad) over the gestational period19-21. However, the International Commission on Radiological Protection (ICRP) recommends a lower limit with occupational radiation exposure to a foetus of <1 mSv (100 mrem) (Table 2)13,22,23.
Reported radiation exposure that has been associated with risk to the child is significantly higher than the recommended limits. An in utero radiation dose exposure >100 mSv is associated with increased risk of malformation and childhood cancer7. Other studies also report the association of low dose radiation exposure with the development of childhood cancer13,24,25. Foetal risk of malformation increases above background levels at radiation doses above 150 mGy26. The first trimester is the period of greatest risk15. Little is known about the effect of radiation exposure during the first 9 to 10 days, between conception and implantation of the egg. Exposure during 18 to 20th day following conception could result in death and expulsion of the ovum. The impact of radiation exposure is best observed during the phase of organogenesis between 20 and 50 days following conception. Doses ranging from 1-2 Gy could result in serious development abnormalities in the foetus including anomalies of the nervous system, eyes, and skeletal system. Radiation exposure after the 50th day following conception could result in intrauterine growth retardation either of the entire body or only the skull and brain10. Since the threshold dose for these deterministic effects is well above that which an invasive or interventional cardiologist would receive under a protective apron, the use of standard radiation protection techniques would result in negligible risk to the foetus.
Comparison of radiation exposures to the foetus with other non-occupational radiation exposures. Radiation exposure is ubiquitous and background radiation is typically 0.75-1 mSV (0.075-0.1 rem) during gestation27. Background cosmic radiation varies geographically. In Denver, CO, USA the average background radiation from cosmic sources is 0.9 mSv per year compared with the Atlantic coastal region where the background radiation is 0.23 mSv per year28,29. Airline travel is another radiation source, which varies based on the length of the flight, the altitude and the latitude. Long flights in studies varied in radiation from 0.003-0.0097 mSv/hr (0.3-0.97 mrem/hr)30. Airline personnel flying 600- 800 hrs/yr are exposed to 2-5 mSv/yr31.
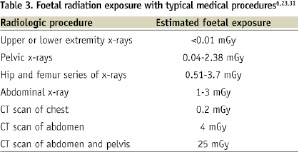
Another potential source of radiation exposure to a foetus is medical imaging. Estimations of foetal doses for common x-rays are <0.01 mGy for upper or lower extremity x-rays but increase to as high as 0.51-3.7 mGy for a hip and femur series of x-rays (Table 3)26.
Foetal dose from helical computed tomography (CT) scans of the abdomen and pelvis has been estimated by simulation studies to be 7.3 to 14.3 mGy/100 milliampere- seconds32. Radiation exposure to the foetus from CT scans also vary based on the imaging field and length of the study, but are as high as 1.52-1.68 mGy at 0 months and 2-4 mGy at three months for an abdominal CT on an appendix protocol33.
Monitoring radiation exposure during pregnancy. To adequately comply with the National Council for Radiation Protection and to ensure the limiting of occupational radiation exposure of the foetus to a value <5 mSv (500 mrem) during pregnancy, monthly monitoring of the radiation exposure under the lead at waist level is typically recommended (Table 5).

Using a personal monitoring dosimeter, radiation exposure down to 0.01 mSv (1 mrem) can be determined. Under lead badge measurements of radiation exposure may also be utilised before pregnancy for a woman to evaluate her own individual risk and the risks to her future children. This would allow a woman to determine if any changes in her practice would be necessary during pregnancy. However, it would be unusual for a pregnant cardiologist to receive more than the maximum 1 mSv allowed under a protective lead apron especially if the woman is also behind a table shield34,35.
Physician issues in procedure type and radiation dose management
Radial arterial access. Routine use of the transradial access for diagnostic coronary angiograms and PCI has gained popularity because of the potential to reduce bleeding and vascular complications and improve patient comfort36,37. However, procedures which utilise radial access historically have been associated with increased radiation exposure38-43. Some non-randomised studies have not shown an increase in radiation exposure with radial arterial access44,45 but a subsequent randomised study demonstrated an increase in operator radiation exposure with the radial arterial access technique45. Radial access has been associated with an increase in air kerma, used as an indicator of skin radiation dose, compared to femoral procedures and remained a strong predictor of increased radiation in the multivariate model46. In a more recent randomised study, the procedure duration was longer with the radial approach and the radiation exposure was modestly increased [median DAP: 38.2 Gycm2 vs. 41.9 Gycm2]41. The increase in radiation exposure occurs not only from the increased procedure time, but also from the operator standing closer to the image intensifier during the procedure. It is also difficult to adequately use radiation safety devices with the radial approach39. In addition, the learning curve for radial procedures is quite steep and may significantly add to the procedure time and increase radiation exposure47-49. Minimising radiation through maximising the distance of the operator to the radiation field and proper shielding techniques are always important but take on greater importance when using the radial technique50. Thus, because of the learning curve, pregnancy would not be an ideal time to initiate routine use of radial arterial procedures.
Peripheral vascular interventions. Peripheral vascular interventions may have increased operator radiation exposure compared to coronary interventions done from a femoral access site because of longer procedure times, greater challenges with shielding, and closer location of the operator to the radiation. There is considerable variability to the radiation exposure reported in the literature from all catheterisation procedures. In peripheral procedures the DAP ranges from 6.7-163 Gycm2 compared to a reported range of 6.2-109 Gycm2 for coronary angiography51. Approximately 90% of the total procedural radiation exposure for peripheral procedures comes from manual-injection digital subtraction angiography (DSA), and therefore the use of a power-injector that allows for distancing of the operator from the radiation source can be a useful technique to reduce operator radiation52.
Other potential exposure in the cardiac catheterisation laboratory. Some cardiac catheterisation laboratories have added a Stereotaxis magnetic navigation system to their equipment to assist in the ability to guide a wire in tortuous vessels53,54. The use of this system does not obviate the need for radiation, and additionally adds the exposure of a magnetic field. Exposure to magnetic fields including magnetic resonance imaging (MRI) and the Stereotaxis system are generally considered safer than radiation. Currently the FDA states that the safety of MRI to the foetus has not been established. However, the currently available human data has failed to demonstrate any adverse effects55-57. Occupational exposure in the catheterisation laboratory is somewhat different. Stereotaxis has a smaller magnetic field than MRI, but chronic exposure such as might be seen with a health care employee has not been adequately studied.
Ways to reduce radiation exposure. The key ways of reducing radiation to pregnant personnel in the catheterisation laboratory are consistent with patient safety goals of minimising patient radiation58. The National Council on Radiation Protection requires that occupational radiation exposure is kept at a level as low as reasonably achievable23. Formal education and training in radiation protection is essential to create awareness of the hazards of radiation among interventional cardiologists58-63. In other countries, such as the United Kingdom it is mandatory that all interventional cardiologists working in the catheter laboratory receive adequate training and obtain a certificate from the Ionising Radiation Medical Exposure Regulations (IRMER) before using radiation imaging equipment in the cardiac catheterisation laboratory. These guidelines and regulations were developed to adequately protect employees from medical radiation exposure. A similar policy is now in place in most, if not all, US hospitals. The majority of occupational radiation exposure is from radiation scatter. The optimal use of radiation safety techniques should be used in all cases regardless of the operator’s pregnancy status (Table 4)64,65.
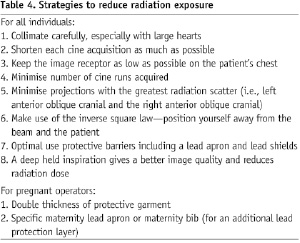
The key protection factors are under the control of the operator using the imaging equipment. The use of these techniques along with optimised lead shields and personal protective equipment can reduce the radiation exposure to 0.8% of unprotected levels66. Lead shields can attenuate at least 99% of the scatter radiation and in studies reduced overall radiation exposure by 50-75%35. Maintaining working views which are postero-anterior (PA) and right anterior oblique (RAO) are preferred to the left anterior oblique (LAO) views as they reduce radiation exposure to the operator standing on the right side of the table67. Increasing the distance of the operator from the x-ray source is important due to the inverse square relationship of dose and distance. It has been demonstrated that increasing the working distance from 40 cm to 80 cm decreases scattered radiation to around one fourth of the original dose68. Likewise, frame rate reduction can significantly impact radiation exposure resulting in a reduction of 40-60% of occupational exposure69,70. This must be balanced however, by the need to obtain adequate, high quality images. Equipment choices, such as digital flat panel systems are also associated with reduced radiation exposure to patients and operators compared with the conventional system71,72. Future innovations, including robotic assisted interventions may lead to dramatic reductions in operator radiation exposure73. Lead or lead-equivalent protective garments are required for x-ray fluoroscopy operators and are vital for radiation attenuation in the cardiac catheterisation laboratory. In a study of 30 operators, the mean projected yearly radiation dose under the protective garments was 0.9 mSv, but was 1.3 mSV for individuals with 0.5-mm lead coverage and 0.4 mSv for those with 1.0-mm lead coverage (P=0.002)74. A 0.25 mm lead apron attenuates 66% of the primary radiation beam at 75 kVp and 55% of the primary beam at 100 kVp, whereas a 0.5 mm lead apron attenuates 88% of the primary beam at 75 kVp and 75% at 100 kVp and a 1 mm thick lead apron attenuates 99% of the primary beam at 75 kVp and 94% at 100 kVp28. However, since the vast majority of radiation exposure to the catheterisation personnel is from scattered radiation, the more relevant information is that a 0.25 mm lead apron absorbs ~96% of scatter radiation while a 0.5 mm lead apron absorbs about 98%75. The NCRP estimates that the conversion from a collar badge reading to the effective dose equivalent under a lead apron can be converted using a factor of 1/5.676. The wrap around style lead skirts offer 0.5 mm lead protection in the front portion, and the sides are 0.25 mm offering reduced protection from angled radiation exposure. Careful attention to the type of lead or non-lead apron and the thickness of the material is important in the assessment of risk. In addition, one must remain observant throughout pregnancy to ensure adequacy of fit and coverage of the apron as improper overlap will result in less effective radiation protection. Pregnant women can utilise standard aprons, and change to a larger size as needed or certain manufacturers make aprons specifically designed for pregnancy which can accommodate the enlarging abdomen. Another technique includes wearing an additional lead apron for double lead coverage over the abdomen. This is equivalent to utilising a thicker apron, and will be as effective as the combined thickness. However, the added weight from the lead would increase the potential for musculoskeletal and back issues which may be seen in pregnancy.
It is important that once a female cardiologist operating in the catheter laboratory becomes pregnant that she not only takes the appropriate measures outlined above but that she informs the proper institutional radiation safety person to ensure that she is adequately monitored throughout her pregnancy. She may also wish to wear a direct reading dosimeter to satisfy herself that day to day radiation exposure is being kept to a minimum.
Current radiation safety practices and beliefs by interventional cardiologists, an SCAI survey
A survey was sent to 9,364 SCAI members and 380 cardiologists responded. Of those who responded, 7% were age 25-34, 27% were 35-44, 36% were 45-54, 24% were 55-64, and 7% were over 65. Of the respondents, 12% were women. Radiation exposure influenced their choice of subspecialty within cardiology in 6%, which is likely underestimating the influence because this survey was taken from cardiologists who went into interventional cardiology. Seventy-six percent reported wearing a collar radiation badge always or most of the time, 8% never wear, and 16% reported occasionally or some of the time wearing a badge. Of these interventionalists, 18% reported not wearing a badge at some point over concerns that they would exceed the radiation limit and 6% reported having had to stop working at some point because of exceeding the radiation limit. Protective equipment including a thyroid collar was used by 94%, lead glasses by 46%, and leg shields by 20%.
This survey demonstrated that 65% of the respondents work where the medical group practice or hospital would allow pregnant women to continue in the cardiac catheterisation laboratory during pregnancy, while 35% stated their practices would not. Of the women who responded and who have had children, only 35% remained in the cardiac catheterisation laboratory performing procedures during pregnancy. It is unclear if this was by personal choice or it was mandated by the practice or the institution. Of the women who performed procedures during pregnancy, 19% wore double lead during the pregnancy. Pregnancy was not declared to the institution by 8%.
The legal rights of the pregnant healthcare worker
In the United States, the Pregnancy Discrimination Act, an amendment to the sex discrimination section of the Civil Rights Act of 1964 was passed in 1978 and was the first law which protected women from employment discrimination based on pregnancy or fertility status77,78. Because of the potential risk of certain occupational exposures, employers continued practices of excluding women who could become pregnant from these occupations79. An example is Johnson Controls, a manufacturer of storage batteries where there was occupation exposure to lead. Since voluntary processes failed to prevent pregnant women from the work area with potential risk to an unborn child, the manufacturer made a policy in 1982 of requiring medical confirmation of the inability to bear children for any women in a job where there was lead exposure. In 1984, a lawsuit of UAW vs. Johnson Controls was brought for discrimination, which eventually made its way to the Supreme Court77. In 1991 the Court ruled that all foetal protection policies are in violation of title VII, and that all exposure protection policies must be applicable to all employees, regardless of pregnancy or the potential to get pregnant. Despite this ruling some hospitals have continued policies prohibiting women from working near radiation when they declare their pregnancy. This policy discourages an employee from disclosing pregnancy status, which protects the institution from any liability for the radiation exposure as the institution has no liability if the pregnancy is not disclosed78. However, this discourages proper monitoring of radiation exposure during pregnancy. In addition, recent court rulings have prohibited these types of policies.
In 2005, the US Equal Opportunity Commission sued Catholic Healthcare West in California for the prevention of a registered nurse and a radiology technician from working around fluoroscopy equipment in the cardiac catheterisation laboratory when they were pregnant. The US District Court for the Central District of California ruled this policy was discriminatory and the hospital now maintains a policy of abiding by the recommendations of the National Council on Radiation Protection and Measurements which limits occupational radiation exposure of the foetus to <5 mSv (500 mrem) during pregnancy.
There is great disparity in the approach to the pregnant healthcare worker in different countries. Italy has one of the strictest positions toward radiation exposure of the pregnant healthcare worker. In Italy, the national law (DL 25/11/1996 number 645-DLgs 26/03/2001 number 151) requires women working with radiation to communicate her pregnancy to the hospital director or the chief of the practice and then the worker is absolutely forbidden to enter the exposed zone throughout the pregnancy.
In Spain, a specific consensus document on pregnancy and hospital practice was created in 2002 on behalf of Consejo de Seguridad Nuclear (the Spanish Council for nuclear safety) and the Spanish Society of Medical Physics. Based on the law where the foetus is considered a public member, the pregnant worker environment must guarantee that the foetus will not receive more than 1 mSv throughout the pregnancy. Currently, the law states that the abdominal radiation dose should be less than 2 mSV and if this is not the case, then a pregnant woman should not work there. Because of the possibility of this occurring in the cardiac catheterisation laboratory, some institutions restrict the work of pregnant women. Nevertheless, the actual radiation dose has a high probability to be less than 2 mSv, which leaves the authorisation to work or not in the catheterisation laboratory at the discretion of the Radiology Protection Office at each institution.
In Japan, based in the Medical Care Act (Article 30-27) the radiation dose to the abdominal regions for pregnant healthcare workers must be less than 2 mSv during the pregnancy. A company monitoring the radiation exposure reported that the average dose to Japanese female physicians was 0.2 mSv/year. Hence, it is believed that the law gives the appropriate safe management for pregnant healthcare workers and the law does not limit their medical practices. However, since interventional cardiologists may have more radiation exposures than other physicians, a survey focused on female interventional cardiologists may still be needed in Japan.
In the United Kingdom, current legislation for occupationally exposed persons and members of the public is contained in IRR99 (Ionising Radiation Regulations 1999) and is based on ICRP 60 (International Commission on Radiological Protection). The most recent ICRP recommendations (ICRP 103) were approved in March 2007. A European Union directive is being drawn up and new legislation is expected in 2015. In IRR99 the dose limit to the abdomen of a woman of reproductive capacity is 13 mSv in any consecutive three months. This could result in a very high foetal dose and in practice almost all hospital radiation workers are unclassified and must not receive more than 3/10ths any personal dose limit. This means their whole body radiation dose must not exceed 6 mSV in any calendar year. Once pregnancy is declared, however, the foetus is then treated as a member of the general public and the radiation dose must be limited to 1 mSv.
In Canada, most catheterisation laboratory personnel are expected to continue working in their usual area, participate in on-call duties, and assist with emergencies during pregnancy. More frequent dosimetry is often available, if the worker so desires. As the pregnancy proceeds, there may be an effort to assign the pregnant worker to more control-room based duties. For pregnant physicians, it is a personal choice with quite variable practice, usually governed by income needs, and mechanical issues or health problems as the pregnancy proceeds.
Throughout the world, there is great variability to the expectations and rights of women to work in the cardiac catheterisation laboratory. Analysis of the risks to the unborn child suggests that in most circumstances the risk to the foetus would be exceptionally small. Careful monitoring of individual risk during pregnancy and adherence to radiation safety protocols to minimise exposure is warranted and should be part of all national and international guidelines.
Conclusion
For a woman to make an informed decision regarding her choices for occupational radiation exposure during pregnancy, she must have a clear understanding of the risk to the foetus. The foetal radiation exposure for most women who work in the cardiac catheterisation laboratory is extremely low, and is far lower than limits recommended by the National Council on Radiation Protection. If a woman wishes to become pregnant and questions her own exposure, she can wear an underlead radiation badge to determine her own exposure before making decisions. Radiation exposure in pregnancy can be significantly reduced by appropriate fit and thickness of lead aprons, radiation shielding, and maximising distance from the radiation source. Thus, based on the available evidence, heritable or developmental risks to the foetus of pregnant interventional cardiology physicians and staff are extremely low provided that good radiation safety practices are used and dose limits are respected. Therefore, concerns over radiation exposure should not be a barrier to choice in pursuing a career in invasive or interventional cardiology, nor should they arbitrarily limit an existing operator’s choices on work environments during pregnancy.
Acknowledgements
The authors thank Elizabeth Schueler and Kenneth Fetterling, Mayo Clinic, Rochester, MN, USA and Rosemary Nicholson, Imperial College Healthcare NHS Trust, London, United Kingdom for their critical review and technical insight of this manuscript.
Conflict of interest
Dr. Best has received speaker’s fees from Medtronic. Dr. Madan is on the Astra Zeneca and Eli Lilly and Company advisory boards; she is a consultant for Astra Zeneca and Eli Lilly and Company; has received research support from Merck and Co. Inc., Pfizer Inc., and Portola Pharmaceuticals; and received speaker’s fees from Eli Lilly and Company, Merck and Co. Inc., Pfizer Inc., and Servier. Dr. Mauri is on the Boston Scientific and Medtronic advisory boards, and received support from Abbott. Dr. Mehran is on the Regado advisory board; she has received research support from Accumetrics and Bracco; and received speaker’s fees from Abiomed, Abbott, Accumetric, AlphaMedica, Astra Zeneca, Bristol-Myers Squibb, Bracco, Cariva, Daiichi Sankyo, Datascope, Gilead, Gerbet, Medtronic, Regado, St. Jude, and Therox. Dr. Mikhail has received support from Abbott Vascular. Dr. Skelding sits on the Medtronic advisory board and has received research support from Abbott and Medtronic. Dr. Weiner is a consultant for Aptus, Atheromed, Atricure, Boston Biomedical Associates, BSC, Capella, GI Dynamics, LabCoat, Therox, and Viacor; she is an employee of Accreditation for Cardiovascular Excellence and Imaging Core Lab Services. Drs. Chieffo, Kunadian, Hernandez-Antolin, Honye, and Takahashi have nothing to report.